Serum circulating sirtuin 6 as a novel predictor of mortality after acute ischemic stroke
- PMID: 36443316
- PMCID: PMC9705558
- DOI: 10.1038/s41598-022-23211-y
Serum circulating sirtuin 6 as a novel predictor of mortality after acute ischemic stroke
Abstract
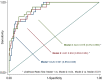
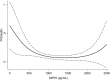
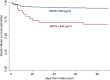
In a murine model of acute ischemic stroke, SIRT6 knockdown resulted in larger cerebral infarct size, worse neurological outcome, and higher mortality, indicating a possible neuro-protective role of SIRT6. In this study, we aimed at evaluating the prognostic value of serum SIRT6 levels in patients with acute ischemic stroke (AIS). Serum levels of SIRT6, collected within 72 h from symptom-onset, were measured in 317 consecutively enrolled AIS patients from the COSMOS cohort. The primary endpoint of this analysis was 90-day mortality. The independent prognostic value of SIRT6 was assessed with multivariate logistic and Cox proportional regression models. 35 patients (11%) deceased within 90-day follow-up. After adjustment for established risk factors (age, NIHSS, heart failure, atrial fibrillation, and C reactive protein), SIRT6 levels were negatively associated with mortality. The optimal cut-off for survival was 634 pg/mL. Patients with SIRT6 levels below this threshold had a higher risk of death in multivariable Cox regression. In this pilot study, SIRT6 levels were significantly associated with 90-day mortality after AIS; these results build on previous molecular and causal observations made in animal models. Should this association be confirmed, SIRT6 could be a potential prognostic predictor and therapeutic target in AIS.
© 2022. The Author(s).
Conflict of interest statement
Dr. Liberale and Prof. Camici are coinventors on the International Patent WO/2020/226993 filed in April 2020; the patent relates to the use of antibodies which specifically bind interleukin-1a to reduce various sequelae of ischemia–reperfusion injury to the central nervous system. Prof. Camici is a consultant to Sovida solutions limited. Dr Liberale has received financial support from the Swiss Heart Foundation and the Novartis Foundation for Medical-Biological Research. Dr. Arnold has received speaker honoraria outside of this work from Daichi-Sankyo. Dr. Arnold reports grants from the Kurt und Senta Hermann Stiftung during the conduct of the study. Dr. Arnold received personal fees from Bayer, BMS, Covidien, Medtronic, Amgen, Daiichi Sankyo, Nestle Health Science, and Boehringer Ingelheim, outside the submitted work. Prof. Christ-Crain received speaker honoraria from Thermo Fisher AG. Prof. Montecucco has received financial support from the “Rete Cardiologica” of Italian Ministry of Health (#2754291). Prof. Camici is the recipient of a Sheikh Khalifa’s Foundation Assistant Professorship at the Faculty of Medicine, University of Zurich. Prof. Katan Kahles reports grants from Spital-Pool of the University Hospital of Zurich and EMDO Foundation during the conduction of the study. Prof. Katan Kahles received non-financial support from Spital-Pool of the University Hospital of Zurich and ROCHE, outside the submitted work. None of the remaining Authors has potential conflict of interest to disclose. All other Authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

