Targeting APLN/APJ restores blood-testis barrier and improves spermatogenesis in murine and human diabetic models
- PMID: 36443325
- PMCID: PMC9705293
- DOI: 10.1038/s41467-022-34990-3
Targeting APLN/APJ restores blood-testis barrier and improves spermatogenesis in murine and human diabetic models
Abstract
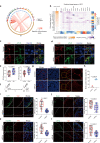
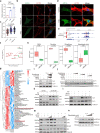
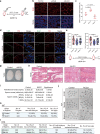
Type 2 diabetes mellitus is one of the most prevalent metabolic diseases presenting with systemic pathologies, including reproductive disorders in male diabetic patients. However, the molecular mechanisms that contributing to spermatogenesis dysfunction in diabetic patients have not yet been fully elucidated. Here, we perform STRT-seq to examine the transcriptome of diabetic patients' testes at single-cell resolution including all major cell types of the testis. Intriguingly, whereas spermatogenesis appears largely preserved, the gene expression profiles of Sertoli cells and the blood-testis barrier (BTB) structure are dramatically impaired. Among these deregulate pathways, the Apelin (APLN) peptide/Apelin-receptor (APJ) axis is hyper-activated in diabetic patients' testes. Mechanistically, APLN is produced locally by Sertoli cells upon high glucose treatment, which subsequently suppress the production of carnitine and repress the expression of cell adhesion genes in Sertoli cells. Together, these effects culminate in BTB structural dysfunction. Finally, using the small molecule APLN receptor antagonist, ML221, we show that blocking APLN/APJ significantly ameliorate the BTB damage and, importantly, improve functional spermatogenesis in diabetic db/db mice. We also translate and validate these findings in cultured human testes. Our findings identify the APLN/APJ axis as a promising therapeutic target to improve reproduction capacity in male diabetic patients.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet. Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

