Terlipressin combined with conservative fluid management attenuates hemorrhagic shock-induced acute kidney injury in rats
- PMID: 36443404
- PMCID: PMC9705717
- DOI: 10.1038/s41598-022-24982-0
Terlipressin combined with conservative fluid management attenuates hemorrhagic shock-induced acute kidney injury in rats
Abstract
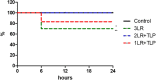
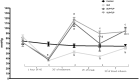
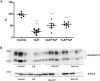
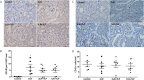
Hemorrhagic shock (HS), a major cause of trauma-related mortality, is mainly treated by crystalloid fluid administration, typically with lactated Ringer's (LR). Despite beneficial hemodynamic effects, such as the restoration of mean arterial pressure (MAP), LR administration has major side effects, including organ damage due to edema. One strategy to avoid such effects is pre-hospitalization intravenous administration of the potent vasoconstrictor terlipressin, which can restore hemodynamic stability/homeostasis and has anti-inflammatory effects. Wistar rats were subjected to HS for 60 min, at a target MAP of 30-40 mmHg, thereafter being allocated to receive LR infusion at 3 times the volume of the blood withdrawn (liberal fluid management); at 2 times the volume (conservative fluid management), plus terlipressin (10 µg/100 g body weight); and at an equal volume (conservative fluid management), plus terlipressin (10 µg/100 g body weight). A control group comprised rats not subjected to HS and receiving no fluid resuscitation or treatment. At 15 min after fluid resuscitation/treatment, the blood previously withdrawn was reinfused. At 24 h after HS, MAP was higher among the terlipressin-treated animals. Terlipressin also improved post-HS survival and provided significant improvements in glomerular/tubular function (creatinine clearance), neutrophil gelatinase-associated lipocalin expression, fractional excretion of sodium, aquaporin 2 expression, tubular injury, macrophage infiltration, interleukin 6 levels, interleukin 18 levels, and nuclear factor kappa B expression. In terlipressin-treated animals, there was also significantly higher angiotensin II type 1 receptor expression and normalization of arginine vasopressin 1a receptor expression. Terlipressin associated with conservative fluid management could be a viable therapy for HS-induced acute kidney injury, likely attenuating such injury by modulating the inflammatory response via the arginine vasopressin 1a receptor.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






Similar articles
-
A comparison of vasopressin, terlipressin, and lactated ringers for resuscitation of uncontrolled hemorrhagic shock in an animal model.PLoS One. 2014 Apr 23;9(4):e95821. doi: 10.1371/journal.pone.0095821. eCollection 2014. PLoS One. 2014. PMID: 24759799 Free PMC article.
-
Vasopressin analog terlipressin attenuates kidney injury in hemorrhagic shock.Trauma Surg Acute Care Open. 2016 Sep 26;1(1):e000039. doi: 10.1136/tsaco-2016-000039. eCollection 2016. Trauma Surg Acute Care Open. 2016. PMID: 29766070 Free PMC article.
-
Can a Therapeutic Strategy for Hypotension Improve Cerebral Perfusion and Oxygenation in an Experimental Model of Hemorrhagic Shock and Severe Traumatic Brain Injury?Neurocrit Care. 2023 Oct;39(2):320-330. doi: 10.1007/s12028-023-01802-5. Epub 2023 Aug 3. Neurocrit Care. 2023. PMID: 37535176
-
Choice of Whole Blood versus Lactated Ringer's Resuscitation Modifies the Relationship between Blood Pressure Target and Functional Outcome after Traumatic Brain Injury plus Hemorrhagic Shock in Mice.J Neurotrauma. 2021 Oct 15;38(20):2907-2917. doi: 10.1089/neu.2021.0157. Epub 2021 Sep 15. J Neurotrauma. 2021. PMID: 34269621 Free PMC article.
-
Hypertonic saline resuscitation enhances blood pressure recovery and decreases organ injury following hemorrhage in acute alcohol intoxicated rodents.J Trauma Acute Care Surg. 2013 Jan;74(1):196-202. doi: 10.1097/TA.0b013e31826fc747. J Trauma Acute Care Surg. 2013. PMID: 23147176 Free PMC article.
Cited by
-
Obesity aggravates acute kidney injury resulting from ischemia and reperfusion in mice.Sci Rep. 2024 Apr 29;14(1):9820. doi: 10.1038/s41598-024-60365-3. Sci Rep. 2024. PMID: 38684767 Free PMC article.
-
Novel model of cardiac hypertrophy with cardiorenal dysfunction.Sci Rep. 2025 Jul 19;15(1):26242. doi: 10.1038/s41598-025-90435-z. Sci Rep. 2025. PMID: 40683937 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

