Cerebral Superficial Siderosis : Etiology, Neuroradiological Features and Clinical Findings
- PMID: 36443509
- PMCID: PMC10220152
- DOI: 10.1007/s00062-022-01231-5
Cerebral Superficial Siderosis : Etiology, Neuroradiological Features and Clinical Findings
Abstract
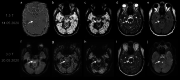
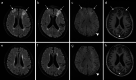
Superficial siderosis (SS) of the central nervous system constitutes linear hemosiderin deposits in the leptomeninges and the superficial layers of the cerebrum and the spinal cord. Infratentorial (i) SS is likely due to recurrent or continuous slight bleeding into the subarachnoid space. It is assumed that spinal dural pathologies often resulting in cerebrospinal fluid (CSF) leakage is the most important etiological group which causes iSS and detailed neuroradiological assessment of the spinal compartment is necessary. Further etiologies are neurosurgical interventions, trauma and arteriovenous malformations. Typical neurological manifestations of this classical type of iSS are slowly progressive sensorineural hearing impairment and cerebellar symptoms, such as ataxia, kinetic tremor, nystagmus and dysarthria. Beside iSS, a different type of SS restricted to the supratentorial compartment can be differentiated, i.e. cortical (c) SS, especially in older people often due to cerebral amyloid angiopathy (CAA). Clinical presentation of cSS includes transient focal neurological episodes or "amyloid spells". In addition, spontaneous and amyloid beta immunotherapy-associated CAA-related inflammation may cause cSS, which is included in the hemorrhagic subgroup of amyloid-related imaging abnormalities (ARIA). Because a definitive diagnosis requires a brain biopsy, knowledge of neuroimaging features and clinical findings in CAA-related inflammation is essential. This review provides neuroradiological hallmarks of the two groups of SS and give an overview of neurological symptoms and differential diagnostic considerations.
Keywords: Amyloid related imaging abnormalities; Cerebral amyloid angiopathy; Cortical; Infratentorial; Superficial siderosis.
© 2022. The Author(s).
Conflict of interest statement
S. Weidauer, E. Neuhaus and E. Hattingen declare that they have no competing interests.
Figures








References
-
- Hamill RC. Report of a case of melanosis of the brain, cord, and meninges. J Nerv Men Dis. 1908;35(9):594. doi: 10.1097/00005053-190809000-00027. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

