Crenigacestat blocking notch pathway reduces liver fibrosis in the surrounding ecosystem of intrahepatic CCA viaTGF-β inhibition
- PMID: 36443822
- PMCID: PMC9703776
- DOI: 10.1186/s13046-022-02536-6
Crenigacestat blocking notch pathway reduces liver fibrosis in the surrounding ecosystem of intrahepatic CCA viaTGF-β inhibition
Abstract
Background: Intrahepatic cholangiocarcinoma (iCCA) is a highly malignant tumor characterized by an intensive desmoplastic reaction due to the exaggerated presence of the extracellular (ECM) matrix components. Liver fibroblasts close to the tumor, activated by transforming growth factor (TGF)-β1 and expressing high levels of α-smooth muscle actin (α-SMA), become cancer-associated fibroblasts (CAFs). CAFs are deputed to produce and secrete ECM components and crosstalk with cancer cells favoring tumor progression and resistance to therapy. Overexpression of Notch signaling is implicated in CCA development and growth. The study aimed to determine the effectiveness of the Notch inhibitor, Crenigacestat, on the surrounding microenvironment of iCCA.
Methods: We investigated Crenigacestat's effectiveness in a PDX model of iCCA and human primary culture of CAFs isolated from patients with iCCA.
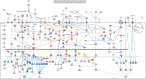
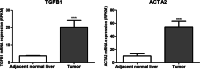
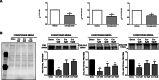
Results: In silico analysis of transcriptomic profiling from PDX iCCA tissues treated with Crenigacestat highlighted "liver fibrosis" as one of the most modulated pathways. In the iCCA PDX model, Crenigacestat treatment significantly (p < 0.001) reduced peritumoral liver fibrosis. Similar results were obtained in a hydrodynamic model of iCCA. Bioinformatic prediction of the upstream regulators related to liver fibrosis in the iCCA PDX treated with Crenigacestat revealed the involvement of the TGF-β1 pathway as a master regulator gene showing a robust connection between TGF-β1 and Notch pathways. Consistently, drug treatment significantly (p < 0.05) reduced TGF-β1 mRNA and protein levels in tumoral tissue. In PDX tissues, Crenigacestat remarkably inhibited TGF-β signaling and extracellular matrix protein gene expression and reduced α-SMA expression. Furthermore, Crenigacestat synergistically increased Gemcitabine effectiveness in the iCCA PDX model. In 31 iCCA patients, TGF-β1 and α-SMA were upregulated in the tumoral compared with peritumoral tissues. In freshly isolated CAFs from patients with iCCA, Crenigacestat significantly (p < 0.001) inhibited Notch signaling, TGF-β1 secretion, and Smad-2 activation. Consequently, Crenigacestat also inactivated CAFs reducing (p < 0.001) α-SMA expression. Finally, CAFs treated with Crenigacestat produced less (p < 005) ECM components such as fibronectin, collagen 1A1, and collagen 1A2.
Conclusions: Notch signaling inhibition reduces the peritumoral desmoplastic reaction in iCCA, blocking the TGF-β1 canonical pathway.
Keywords: Crenigacestat; Liver fibrosis; Smad2; Tissue microenvironment; Tumor stroma crosstalk.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




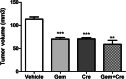



References
-
- Khan SA, Tavolari S, Brandi G. Cholangiocarcinoma: epidemiology and risk factors. Liver Int. 2019;39 Suppl 1:19–31. - PubMed

