Intra-articular injection choice for osteoarthritis: making sense of cell source-an updated systematic review and dual network meta-analysis
- PMID: 36443838
- PMCID: PMC9703652
- DOI: 10.1186/s13075-022-02953-0
Intra-articular injection choice for osteoarthritis: making sense of cell source-an updated systematic review and dual network meta-analysis
Abstract
Background: Intra-articular injection is indicated for mild or moderate osteoarthritis (OA). However, the superiority of cell-based injection and the role of diverse cell sources are still unclear. This study aimed to compare the therapeutic effect of intra-articular injection with mesenchymal stem cells (MSCs) and cell-free methods for OA treatment.
Methods: A literature search of published scientific data was carried out from PubMed, MEDLINE, Embase, Cochrane Library, Web of Science, and China National Knowledge Internet (CNKI). Randomized controlled trials (RCTs) compared the efficacy and safety of MSC and cell-free intra-articular injection treatments for OA with at least 6-month follow-up.
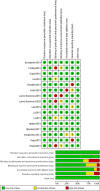
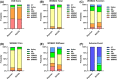
Results: Dual network meta-analysis validated the therapeutic advantages of MSC treatments (VAS, Bayesian: 90% versus 10% and SUCRA: 94.9% versus 5.1%; WOMAC total, Bayesian: 83% versus 17% and SUCRA: 90.1% versus 9.9%) but also suggested a potential negative safety induced by cell injection (adverse events, Bayesian: 100% versus 0% and SUCRA: 98.2% versus 1.8%). For the MSC source aspect, adipose mesenchymal stem cells (ADMSCs) and umbilical cord mesenchymal stem cells (UBMSCs) showed a better curative effect on pain relief and function improvement compared with bone marrow mesenchymal stem cells (BMMSCs).
Conclusion: Intra-articular injection of MSCs is associated with more effective pain alleviation and function improvement than cell-free OA treatment. However, the potential complications induced by MSCs should be emphasized. A comparative analysis of the MSC sources showed that ADMSCs and UBMSCs exerted a better anti-arthritic efficacy than BMMSCs. Schematic illustration of MSC-based intra-articular injection for treating OA. Three major MSCs (UBMSCs, ADMSCs, and BMMSCs) are extracted and expanded in vitro. Subsequently, the amplified MSCs are concentrated and injected into the knee joint to treat OA.
Keywords: Adverse effects; Function improvement; Intra-articular injection; Mesenchymal stem cells; Osteoarthritis; Pain relief.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interest.
Figures



References
-
- Bahrami MH, Raeissadat SA, Cheraghi M, Rahimi-Dehgolan S, Ebrahimpour A. Efficacy of single high-molecular-weight versus triple low-molecular-weight hyaluronic acid intra-articular injection among knee osteoarthritis patients. BMC Musculoskelet Disord. 2020;21(1):550. doi: 10.1186/s12891-020-03577-8. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

