Outcomes by Cardiac Stage in Patients With Newly Diagnosed AL Amyloidosis: Phase 3 ANDROMEDA Trial
- PMID: 36444227
- PMCID: PMC9700253
- DOI: 10.1016/j.jaccao.2022.08.011
Outcomes by Cardiac Stage in Patients With Newly Diagnosed AL Amyloidosis: Phase 3 ANDROMEDA Trial
Abstract
Background: Patients with amyloid light chain amyloidosis and severe cardiac dysfunction have a poor prognosis. Treatment options that induce rapid and deep hematologic and organ responses, irrespective of cardiac involvement, are needed.
Objectives: The aim of this study was to evaluate the impact of baseline cardiac stage on efficacy and safety outcomes in the phase 3 ANDROMEDA trial.
Methods: Rates of overall complete hematologic response and cardiac and renal response at 6 months and median major organ deterioration-progression-free survival and major organ deterioration-event-free survival were compared across cardiac stages (I, II, or IIIA) and treatments (daratumumab, bortezomib, cyclophosphamide, and dexamethasone [D-VCd] or bortezomib, cyclophosphamide, and dexamethasone [VCd]). Rates of adverse events (AEs) were summarized for patients with and without baseline cardiac involvement and by cardiac stage.
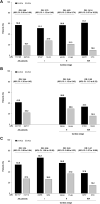
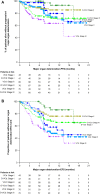
Results: Median follow-up duration was 15.7 months. The proportions of stage I, II, and IIIA patients were 23.2%, 40.2%, and 36.6%. Across cardiac stages, hematologic and organ response rates were higher and major organ deterioration-progression-free survival and major organ deterioration-event-free survival were longer with D-VCd than VCd. AE rates were similar between treatments and by cardiac stage; serious AE rates were higher in patients with cardiac involvement and increased with increasing cardiac stage. The incidence of cardiac events was numerically greater with D-VCd vs VCd, but the rate of grade 3 or 4 events was similar. The exposure-adjusted incidence rate for cardiac events was lower with D-VCd than VCd (median exposure 13.4 and 5.3 months, respectively).
Conclusions: These findings demonstrate the efficacy of D-VCd over VCd in patients with newly diagnosed amyloid light chain amyloidosis across cardiac stages, thus supporting its use in patients with cardiac involvement. (NCT03201965).
Keywords: AE, adverse event; AL, amyloid light chain; CR, complete response; D-VCd, daratumumab, bortezomib, cyclophosphamide, and dexamethasone; EFS, event-free survival; FLC, free light chain; Mayo staging system; NT-proBNP, N-terminal pro–brain natriuretic peptide; NYHA, New York Heart Association; PFS, progression-free survival; SAE, serious adverse event; VCd, bortezomib, cyclophosphamide, and dexamethasone; daratumumab; hs-cTnT, high-sensitivity cardiac troponin T.
© 2022 The Authors.
Conflict of interest statement
This research was funded by Janssen Research & Development. Prof Minnema has consulted or served in an advisory role for Janssen-Cilag, Alnylam, and Gilead; has served on a Speakers Bureau for Bristol Myers Squibb; and has received travel, accommodation, and expense compensation from Celgene. Dr Dispenzieri has received research funding from Alnylam, Celgene, Intellia, Janssen, Pfizer, and Takeda. Dr Comenzo has consulted or served in an advisory role for Amgen, Caelum, Janssen, Karyopharm, Prothena, Sanofi, Takeda, and Unum; and has received research funding from Janssen, Karyopharm, Prothena, and Takeda. Dr Kastritis has consulted or served in an advisory role for and has received honoraria from Amgen, Genesis Pharma, Janssen, Pfizer, and Takeda; and has received research funding from Amgen and Janssen. Prof Wechalekar has consulted or served in an advisory role for Caelum and Janssen; has received honoraria from Celgene, Janssen, and Takeda; and has received travel, accommodation, and expense compensation from Takeda. Dr Witteles has served in an advisory role for Alnylam Pharmaceuticals, Pfizer, Akcea/Ionis, Eidos, and Regeneron. Dr Ruberg has consulted for Attralus and Alexion; and has received research funding from Akcea, Alnylam, and Pfizer. Dr Maurer has consulted or served in an advisory role for Alnylam Pharmaceuticals and Pfizer; and has received research funding from Akcea, Alnylam Pharmaceuticals, Eidos, Ionis, Intellia, Novo Nordisk, and Pfizer. Prof Jaccard has consulted or served in an advisory role for Janssen; and has received honoraria, research funding, and travel, accommodation, and expense compensation from Celgene and Janssen. Dr Weiss was an employee of Janssen at the time the study was conducted. Dr Tran, Mr Qin, Ms Vasey, and Dr Vermeulen are employees of Janssen. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures



References
-
- Muchtar E., Dispenzieri A., Magen H., et al. Systemic amyloidosis from A (AA) to T (ATTR): a review. J Intern Med. 2020;289:268–292. - PubMed
-
- Chaulagain C.P., Comenzo R.L. New insights and modern treatment of AL amyloidosis. Curr Hematol Malig Rep. 2013;8(4):291–298. - PubMed
-
- Wechalekar A.D., Schonland S.O., Kastritis E., et al. A European collaborative study of treatment outcomes in 346 patients with cardiac stage III AL amyloidosis. Blood. 2013;121(17):3420–3427. - PubMed
-
- Palladini G., Milani P., Merlini G. Novel strategies for the diagnosis and treatment of cardiac amyloidosis. Expert Rev Cardiovasc Ther. 2015;13(11):1195–1211. - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

