AL Amyloidosis for Cardiologists: Awareness, Diagnosis, and Future Prospects: JACC: CardioOncology State-of-the-Art Review
- PMID: 36444232
- PMCID: PMC9700258
- DOI: 10.1016/j.jaccao.2022.08.009
AL Amyloidosis for Cardiologists: Awareness, Diagnosis, and Future Prospects: JACC: CardioOncology State-of-the-Art Review
Abstract
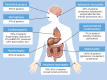
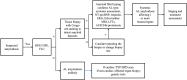
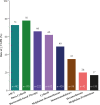
Amyloid light chain (AL) amyloidosis is a rare, debilitating, often fatal disease. Symptoms of cardiomyopathy are common presenting features, and patients often are referred to cardiologists. Cardiac amyloid infiltration is the leading predictor of death. However, the variable presentation and perceived rarity of the disease frequently lead to delay in suspecting amyloidosis as a cause of heart failure, leading to misdiagnoses and a marked delay in diagnosis, with devastating consequences for the patient. A median time from symptom onset to correct diagnosis of about 2 years is often too long when median survival from diagnosis for patients with AL amyloidosis and cardiomyopathy is 4 months to 2 years. The authors highlight the challenges to diagnosis, identify gaps in the current knowledge, and summarize novel treatments on the horizon to raise awareness about the critical need for early recognition of symptoms and diagnosis of AL amyloidosis aimed at accelerating treatment and improving outcomes for patients.
Keywords: AL amyloidosis; AL, amyloid light chain; ASCT, autologous stem cell transplantation; ATTR, transthyretin; CMR, cardiac magnetic resonance imaging; CR, complete response; CyBorD, cyclophosphamide-bortezomib-dexamethasone; FLC, free light chain; Ig, immunoglobulin; LGE, late gadolinium enhancement; NT-proBNP, N-terminal pro–brain natriuretic peptide; PCD, plasma cell dyscrasia; QoL, quality of life; VGPR, very good partial response; awareness; diagnosis; future therapies.
© 2022 The Authors.
Conflict of interest statement
Support for the development of this paper was provided by Alexion, AstraZeneca Rare Disease. Drs Wechalekar, Fontana, and Liedtke have received trial support from Alexion, AstraZeneca Rare Disease. Dr Quarta is an employee of Alexion and AstraZeneca Rare Disease, and has stock in the company.
Figures



References
-
- Vaxman I., Gertz M. Recent advances in the diagnosis, risk stratification, and management of systemic light-chain amyloidosis. Acta Haematol. 2019;141:93–106. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

