Microdevice-based mechanical compression on living cells
- PMID: 36444299
- PMCID: PMC9699986
- DOI: 10.1016/j.isci.2022.105518
Microdevice-based mechanical compression on living cells
Abstract
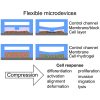
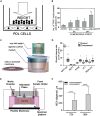
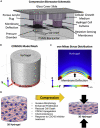
Compressive stress enables the investigation of a range of cellular processes in which forces play an important role, such as cell growth, differentiation, migration, and invasion. Such solid stress can be introduced externally to study cell response and to mechanically induce changes in cell morphology and behavior by static or dynamic compression. Microfluidics is a useful tool for this, allowing one to mimic in vivo microenvironments in on-chip culture systems where force application can be controlled spatially and temporally. Here, we review the mechanical compression applications on cells with a broad focus on studies using microtechnologies and microdevices to apply cell compression, in comparison to off-chip bulk systems. Due to their unique features, microfluidic systems developed to apply compressive forces on single cells, in 2D and 3D culture models, and compression in cancer microenvironments are emphasized. Research efforts in this field can help the development of mechanoceuticals in the future.
Keywords: Mechanobiology; biological sciences; biophysics; cell biology.
© 2022 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Kim Y.C., Kang J.H., Park S.J., Yoon E.S., Park J.K. Microfluidic biomechanical device for compressive cell stimulation and lysis. Sens. Actuators B Chem. 2007;128:108–116. doi: 10.1016/j.snb.2007.05.050. - DOI
-
- Kurth F., Eyer K., Franco-Obregón A., Dittrich P.S. A new mechanobiological era: microfluidic pathways to apply and sense forces at the cellular level. Curr. Opin. Chem. Biol. 2012;16:400–408. doi: 10.1016/j.cbpa.2012.03.014. https://www.sciencedirect.com/science/article/pii/S136759311200049X - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

