Chromodomain helicase DNA-binding domain 2 maintains spermatogonial self-renewal by promoting chromatin accessibility and mRNA stability
- PMID: 36444304
- PMCID: PMC9700024
- DOI: 10.1016/j.isci.2022.105552
Chromodomain helicase DNA-binding domain 2 maintains spermatogonial self-renewal by promoting chromatin accessibility and mRNA stability
Abstract
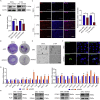
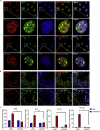
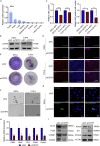
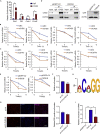
Chromodomain helicase DNA-binding domain 2 (CHD2) is a chromatin remodeling factor involved in many developmental processes. However, its role in male germ cell development has not been elucidated. Here, we confirm that CHD2 expression is enriched in the male germline. In a heterozygous knockout mouse model of Chd2 (Chd2 +/-), we demonstrated that Chd2 haploinsufficiency resulted in testicular developmental delay, an increased rate of abnormal sperm, and impaired fertility in mice. In vitro experiments in mouse spermatogonia showed that CHD2 knockdown inhibits spermatogonial self-renewal. Mechanistically, CHD2 maintains the enrichment of H3K4me3 in the Ccnb1 and Ccnd2 promotors, consequently promoting the transcription of Ccnb1 and Ccnd2. In addition, CHD2 interacts with the cleavage stimulation factor CSTF3 and upregulates the expression of OCT4 and PLZF by improving mRNA stability. This is the first study to reveal the role and mechanism of CHD2 in maintaining spermatogonial self-renewal.
Keywords: Biological sciences; alternate contact; cell biology; molecular mechanism of gene regulation; transcriptomics.
© 2022 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
CHD4 acts as a critical regulator in the survival of spermatogonial stem cells in mice†.Biol Reprod. 2022 Nov 14;107(5):1331-1344. doi: 10.1093/biolre/ioac162. Biol Reprod. 2022. PMID: 35980806
-
Disruption of chromodomain helicase DNA binding protein 2 (CHD2) causes scoliosis.Am J Med Genet A. 2008 May 1;146A(9):1117-27. doi: 10.1002/ajmg.a.32178. Am J Med Genet A. 2008. PMID: 18386809 Free PMC article.
-
Regulation of human cortical interneuron development by the chromatin remodeling protein CHD2.Sci Rep. 2022 Sep 17;12(1):15636. doi: 10.1038/s41598-022-19654-y. Sci Rep. 2022. PMID: 36115870 Free PMC article.
-
Chromatin Remodeling Proteins in Epilepsy: Lessons From CHD2-Associated Epilepsy.Front Mol Neurosci. 2018 Jun 15;11:208. doi: 10.3389/fnmol.2018.00208. eCollection 2018. Front Mol Neurosci. 2018. PMID: 29962935 Free PMC article. Review.
-
CHD2-Related CNS Pathologies.Int J Mol Sci. 2021 Jan 8;22(2):588. doi: 10.3390/ijms22020588. Int J Mol Sci. 2021. PMID: 33435571 Free PMC article. Review.
Cited by
-
Chromatin remodeler CHD8 is required for spermatogonial proliferation and early meiotic progression.Nucleic Acids Res. 2024 Apr 12;52(6):2995-3010. doi: 10.1093/nar/gkad1256. Nucleic Acids Res. 2024. PMID: 38224953 Free PMC article.
-
Integration of Microarray and Single-Cell RNA-Seq Data and Machine Learning Allows the Identification of Key Histone Modification Gene Changes in Spermatogonial Stem Cells.Biology (Basel). 2025 Apr 8;14(4):387. doi: 10.3390/biology14040387. Biology (Basel). 2025. PMID: 40282252 Free PMC article.
References
-
- Izadyar F., Wong J., Maki C., Pacchiarotti J., Ramos T., Howerton K., Yuen C., Greilach S., Zhao H.H., Chow M., et al. Identification and characterization of repopulating spermatogonial stem cells from the adult human testis. Hum. Reprod. 2011;26:1296–1306. doi: 10.1093/humrep/der026. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

