Italian Real-Life Experience on the Use of Mogamulizumab in Patients with Cutaneous T-Cell Lymphomas
- PMID: 36444356
- PMCID: PMC9700436
- DOI: 10.2147/CMAR.S377015
Italian Real-Life Experience on the Use of Mogamulizumab in Patients with Cutaneous T-Cell Lymphomas
Abstract
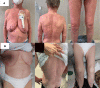
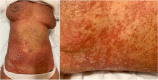
Mycosis fungoides and Sèzary syndrome are the most studied subtypes common cutaneous T-cell lymphomas. The current treatment objective is to improve the clinical manifestations of the disease in the affected areas, to relieve symptoms and to halt disease progression. Patients with early-stage mycosis fungoides are usually managed with skin-directed therapies, whereas patients with resistant or advanced-stage mycosis fungoides or Sèzary syndrome often require systemic drugs. Over the last decade, new drugs have been developed, increasing the breadth of treatment options for cutaneous T-cell lymphomas patients. Mogamulizumab is a first-in-class defucosylated humanized IgG1 κ monoclonal antibody, which exerts its anti-tumour action by selectively binding to C-C chemokine receptor 4 and increasing antibody-dependent cellular cytotoxicity activity against malignant T-cells. Several clinical trials showed that mogamulizumab is able to effectively control the cutaneous T-cell lymphomas in each site (skin, blood, lymph nodes and viscera), improving patients' symptoms, function and overall quality of life with a manageable safety profile. In this report, we discuss 12 cases of patients with mycosis fungoides or Sèzary syndrome successfully treated with mogamulizumab in real-life clinical practice in Italy.
Keywords: Sèzary syndrome; cutaneous T- cell lymphoma; mogamulizumab; mycosis fungoides.
© 2022 Caruso et al.
Conflict of interest statement
Pier Luigi Zinzani received consultant fees from MSD, Eusapharma and Novartis; speaker fees from Celltrion, Gilead, Janssen-Cilag, BMS, Servier, MSD, TG Therap, Takeda, Roche, Eusapharma, Kyowa Kirin, Novartis, Incyte and Beigene; Advisory Board fees from Secura-Bio, Celltrion, Gilead, Janssen-Cilag, BMS, Servier, Sandoz, MSD, TG Therap, Takeda, Roche, Eusapharma, Kyowa Kirin, Novartis, ADC Therapy, Incyte and Beigene. Pietro Quaglino received speaker and advisory board fees from Kyowa Kirin, Takeda, Therakos Cellgene, Helsinn, Recordati, 4 SC. Cesare Massone received speaker and advisory board fees from Kyowa Kirin, Takeda. The other authors report no conflicts of interest in this work.
Figures


Similar articles
-
Cutaneous T-cell lymphomas: a real-life experience of anticipated use of mogamulizumab in Italy.Ital J Dermatol Venerol. 2025 Apr;160(2):97-108. doi: 10.23736/S2784-8671.25.08110-1. Epub 2025 Mar 5. Ital J Dermatol Venerol. 2025. PMID: 40042219
-
Mogamulizumab in the treatment of advanced mycosis fungoides and Sézary syndrome: safety and efficacy.Expert Rev Anticancer Ther. 2020 Jun;20(6):447-452. doi: 10.1080/14737140.2020.1760096. Epub 2020 Apr 28. Expert Rev Anticancer Ther. 2020. PMID: 32320304 Review.
-
Spotlight on Mogamulizumab-Kpkc for Use in Adults with Relapsed or Refractory Mycosis Fungoides or Sézary Syndrome: Efficacy, Safety, and Patient Selection.Drug Des Devel Ther. 2020 Sep 16;14:3747-3754. doi: 10.2147/DDDT.S185896. eCollection 2020. Drug Des Devel Ther. 2020. PMID: 32982179 Free PMC article. Review.
-
Overall survival in the UK in mycosis fungoides or Sézary syndrome cutaneous T-cell lymphoma: comparative effectiveness of mogamulizumab versus current standard of care.J Comp Eff Res. 2023 Oct;12(10):e230017. doi: 10.57264/cer-2023-0017. Epub 2023 Aug 29. J Comp Eff Res. 2023. PMID: 37642410 Free PMC article.
-
Mycosis fungoides and Sézary syndrome: focus on the current treatment scenario.An Bras Dermatol. 2021 Jul-Aug;96(4):458-471. doi: 10.1016/j.abd.2020.12.007. Epub 2021 May 28. An Bras Dermatol. 2021. PMID: 34053802 Free PMC article. Review.
Cited by
-
Atypical Presentation of Invasive Aspergillosis during Treatment with Mogamulizumab.J Fungi (Basel). 2024 Aug 17;10(8):584. doi: 10.3390/jof10080584. J Fungi (Basel). 2024. PMID: 39194909 Free PMC article.
-
A Narrative Review of the State of the Art of CCR4-Based Therapies in Cutaneous T-Cell Lymphomas: Focus on Mogamulizumab and Future Treatments.Antibodies (Basel). 2024 Apr 22;13(2):32. doi: 10.3390/antib13020032. Antibodies (Basel). 2024. PMID: 38804300 Free PMC article. Review.
-
Histopathological Markers for Target Therapies in Primary Cutaneous Lymphomas.Cells. 2023 Nov 20;12(22):2656. doi: 10.3390/cells12222656. Cells. 2023. PMID: 37998391 Free PMC article. Review.
-
Clinical and Real-World Effectiveness of Mogamulizumab: A Narrative Review.Int J Mol Sci. 2024 Feb 12;25(4):2203. doi: 10.3390/ijms25042203. Int J Mol Sci. 2024. PMID: 38396877 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources

