Latent functional diversity may accelerate microbial community responses to temperature fluctuations
- PMID: 36444646
- PMCID: PMC9708066
- DOI: 10.7554/eLife.80867
Latent functional diversity may accelerate microbial community responses to temperature fluctuations
Abstract
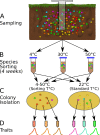
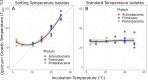
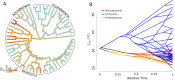
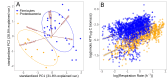
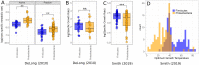
How complex microbial communities respond to climatic fluctuations remains an open question. Due to their relatively short generation times and high functional diversity, microbial populations harbor great potential to respond as a community through a combination of strain-level phenotypic plasticity, adaptation, and species sorting. However, the relative importance of these mechanisms remains unclear. We conducted a laboratory experiment to investigate the degree to which bacterial communities can respond to changes in environmental temperature through a combination of phenotypic plasticity and species sorting alone. We grew replicate soil communities from a single location at six temperatures between 4°C and 50°C. We found that phylogenetically and functionally distinct communities emerge at each of these temperatures, with K-strategist taxa favored under cooler conditions and r-strategist taxa under warmer conditions. We show that this dynamic emergence of distinct communities across a wide range of temperatures (in essence, community-level adaptation) is driven by the resuscitation of latent functional diversity: the parent community harbors multiple strains pre-adapted to different temperatures that are able to 'switch on' at their preferred temperature without immigration or adaptation. Our findings suggest that microbial community function in nature is likely to respond rapidly to climatic temperature fluctuations through shifts in species composition by resuscitation of latent functional diversity.
Keywords: bacteria; diversity; ecology; temperature; thermal response.
Plain language summary
Most ecosystems on Earth rely on dynamic communities of microorganisms which help to cycle nutrients in the environment. There is increasing concern that climate change may have a profound impact on these complex networks formed of large numbers of microbial species linked by intricate biochemical relationships. Any species within a microbial community can acclimate to new temperatures by quickly tweaking their biological processes, for example by activating genes that are more suited to warmer conditions. Over time, a species may acclimate or adapt to new conditions. However, the community as a whole can also respond to these changes, and often much faster, by simply altering the abundance or presence of its members through a process known as species sorting. It remains unclear exactly how acclimation, adaptation and species sorting each contribute to the community’s response to a temperature shift – an increasingly common scenario under global climate change. To address this question, Smith et al. investigated how species sorting and acclimation may help whole soil bacterial communities to cope with lasting changes in temperature. To do so, soil samples from a single field site (and therefore featuring the same microbial community) were incubated for four weeks under six different temperatures. Genetic analyses revealed that, at the end of the experiments, distinct communities specific to a given temperature had emerged. They all differed in species composition and the types of biological functions they could perform. Further experiments showed that each community had been taken over by strains of bacteria which grew best at the new temperature that they had been exposed to, including extreme warming scenarios never seen in their native environment. This suggests that these organisms were already present in the original community. They had persisted even under temperatures which were not optimal for them, acting as a slumbering (‘latent’) ‘reservoir’ of traits and functional abilities that allowed species sorting to produce distinct and functionally capable communities in each novel thermal environment. This suggests that species sorting could help bacterial communities to cope with dramatic changes in their thermal environment. Smith et al.’s findings suggest that bacterial communities can cope with warming environments much better than has been previously thought. In the future, this work may help researchers to better predict how climate change could impact microbial community structure and functioning, and most crucially their contributions to the global carbon cycle.
© 2022, Smith et al.
Conflict of interest statement
TS, SM, ER, DK, SP, TB No competing interests declared
Figures
References
-
- Angilletta MJ. Thermal acclimation. In Thermal Adaption: A Theoretical and Empirical Synthesis; 2009. pp. 126–156.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

