Fixation can change the appearance of phase separation in living cells
- PMID: 36444977
- PMCID: PMC9817179
- DOI: 10.7554/eLife.79903
Fixation can change the appearance of phase separation in living cells
Abstract
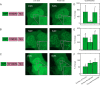
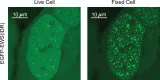
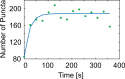
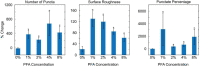
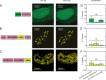
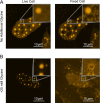
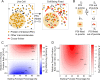
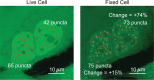
Fixing cells with paraformaldehyde (PFA) is an essential step in numerous biological techniques as it is thought to preserve a snapshot of biomolecular transactions in living cells. Fixed-cell imaging techniques such as immunofluorescence have been widely used to detect liquid-liquid phase separation (LLPS) in vivo. Here, we compared images, before and after fixation, of cells expressing intrinsically disordered proteins that are able to undergo LLPS. Surprisingly, we found that PFA fixation can both enhance and diminish putative LLPS behaviors. For specific proteins, fixation can even cause their droplet-like puncta to artificially appear in cells that do not have any detectable puncta in the live condition. Fixing cells in the presence of glycine, a molecule that modulates fixation rates, can reverse the fixation effect from enhancing to diminishing LLPS appearance. We further established a kinetic model of fixation in the context of dynamic protein-protein interactions. Simulations based on the model suggest that protein localization in fixed cells depends on an intricate balance of protein-protein interaction dynamics, the overall rate of fixation, and notably, the difference between fixation rates of different proteins. Consistent with simulations, live-cell single-molecule imaging experiments showed that a fast overall rate of fixation relative to protein-protein interaction dynamics can minimize fixation artifacts. Our work reveals that PFA fixation changes the appearance of LLPS from living cells, presents a caveat in studying LLPS using fixation-based methods, and suggests a mechanism underlying the fixation artifact.
Keywords: cell biology; cross-linking; fixation; intrinsically disordered proteins; liquid–liquid phase separation; live-cell single-molecule imaging; multivalent protein–protein interactions; none; paraformaldehyde; physics of living systems.
Plain language summary
A typical human cell is a crowded soup of thousands of different proteins. One way that the cell organizes this complex mix of contents is by creating separate droplets within the cell, like oil in water. These droplets can form through a process known as liquid-liquid phase separation, or LLPS, where specific proteins gather in high concentrations to carry out their cellular roles. The critical role of LLPS in cellular organization means that it is widely studied by biologists. To detect LLPS, researchers often subject the cells to treatments designed to hold all the proteins in place, creating a snapshot of their natural state. This process, known as fixing, allows scientists to easily label a protein with a fluorescent tag, take pictures of the cells, and look at whether the protein forms droplets in its natural state. This is often easier to do than imaging cells live, but it relies on LLPS being well-preserved upon fixation. To test if this is true, Irgen-Gioro, Yoshida et al. looked at protein droplets in live cells, and then fixed the cells to check whether the appearance of the droplets had changed. The images taken showed that fixation could alter the size and number of droplets depending on the protein being studied. To explain why the effects of fixing change depending on the protein, Irgen-Gioro, Yoshida et al. hypothesized that a faster fixation – relative to how quickly proteins can bind and unbind to their droplets – can better preserve the LLPS droplets. They verified their idea using a microscopy technique in which they imaged single molecules, allowing them to see how different fixation speeds relative to protein binding affected the droplets. The work of Irgen-Gioro, Yoshida et al. identifies an important caveat to using fixation for the study of LLPS in cells. Their findings suggest that researchers should be cautious when interpreting the results of such studies. Given that LLPS in cells is an area of research with a lot of interest, these results could benefit a broad range of biological and medical fields. In the future, Irgen-Gioro, Yoshida et al.’s findings could prompt scientists to develop new fixing methods that better preserve LLPS in cells.
© 2022, Irgen-Gioro, Yoshida et al.
Conflict of interest statement
SI, SY, VW, SC No competing interests declared
Figures






Comment in
-
When fixation creates fiction.Elife. 2023 Feb 16;12:e85671. doi: 10.7554/eLife.85671. Elife. 2023. PMID: 36795466 Free PMC article.
Similar articles
-
Ubiquitin Modulates Liquid-Liquid Phase Separation of UBQLN2 via Disruption of Multivalent Interactions.Mol Cell. 2018 Mar 15;69(6):965-978.e6. doi: 10.1016/j.molcel.2018.02.004. Epub 2018 Mar 8. Mol Cell. 2018. PMID: 29526694 Free PMC article.
-
Biological soft matter: intrinsically disordered proteins in liquid-liquid phase separation and biomolecular condensates.Essays Biochem. 2022 Dec 16;66(7):831-847. doi: 10.1042/EBC20220052. Essays Biochem. 2022. PMID: 36350034 Review.
-
Phase Separation of Epstein-Barr Virus EBNA2 and Its Coactivator EBNALP Controls Gene Expression.J Virol. 2020 Mar 17;94(7):e01771-19. doi: 10.1128/JVI.01771-19. Print 2020 Mar 17. J Virol. 2020. PMID: 31941785 Free PMC article.
-
Single-Molecule Measurement of Protein Interaction Dynamics within Biomolecular Condensates.J Vis Exp. 2024 Jan 5;(203). doi: 10.3791/66169. J Vis Exp. 2024. PMID: 38251748
-
The (un)structural biology of biomolecular liquid-liquid phase separation using NMR spectroscopy.J Biol Chem. 2020 Feb 21;295(8):2375-2384. doi: 10.1074/jbc.REV119.009847. Epub 2020 Jan 7. J Biol Chem. 2020. PMID: 31911439 Free PMC article. Review.
Cited by
-
Handling Difficult Cryo-ET Samples: A Study with Primary Neurons from Drosophila melanogaster.Microsc Microanal. 2023 Dec 21;29(6):2127-2148. doi: 10.1093/micmic/ozad125. Microsc Microanal. 2023. PMID: 37966978 Free PMC article.
-
Repair Foci as Liquid Phase Separation: Evidence and Limitations.Genes (Basel). 2022 Oct 13;13(10):1846. doi: 10.3390/genes13101846. Genes (Basel). 2022. PMID: 36292731 Free PMC article. Review.
-
Dynamics of RNA localization to nuclear speckles are connected to splicing efficiency.Sci Adv. 2024 Oct 18;10(42):eadp7727. doi: 10.1126/sciadv.adp7727. Epub 2024 Oct 16. Sci Adv. 2024. PMID: 39413186 Free PMC article.
-
Lab-on-a-chip device for microfluidic trapping and TIRF imaging of single cells.Biomed Microdevices. 2025 Mar 14;27(1):12. doi: 10.1007/s10544-025-00739-0. Biomed Microdevices. 2025. PMID: 40085359 Free PMC article.
-
Microglial-derived C1q integrates into neuronal ribonucleoprotein complexes and impacts protein homeostasis in the aging brain.Cell. 2024 Aug 8;187(16):4193-4212.e24. doi: 10.1016/j.cell.2024.05.058. Epub 2024 Jun 27. Cell. 2024. PMID: 38942014 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

