Grass Carp Reovirus Induces Formation of Lipid Droplets as Sites for Its Replication and Assembly
- PMID: 36445081
- PMCID: PMC9765412
- DOI: 10.1128/mbio.02297-22
Grass Carp Reovirus Induces Formation of Lipid Droplets as Sites for Its Replication and Assembly
Abstract
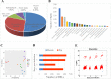
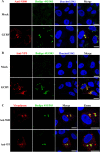
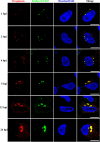
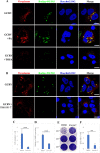
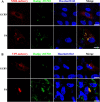
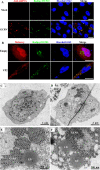
Grass carp is an important commercial fish in China that is plagued by various diseases, especially the hemorrhagic disease induced by grass carp reovirus (GCRV). Nevertheless, the mechanism by which GCRV hijacks the host metabolism to complete its life cycle is unclear. In this study, we performed lipidomic analysis of grass carp liver samples collected before and after GCRV infection. GCRV infection altered host lipid metabolism and increased de novo fatty acid synthesis. Increased de novo fatty acid synthesis induced accumulation of lipid droplets (LDs). LDs are associated with GCRV viroplasms, as well as viral proteins and double-stranded RNA. Pharmacological inhibition of LD formation led to the disappearance of viroplasms, accompanied by decreased viral replication capacity. Moreover, transmission electron microscopy revealed LDs in close association with the viroplasms and mounted GCRV particles. Collectively, these data suggest that LDs are essential for viroplasm formation and are sites for GCRV replication and assembly. Our results revealed the detailed molecular events of GCRV hijacking host lipid metabolism to benefit its replication and assembly, which may provide new perspective for the prevention and control of GCRV. IMPORTANCE Grass carp reovirus (GCRV) is the most virulent pathogen in the genus Aquareovirus, which belongs to the family Reoviridae. GCRV-induced hemorrhagic disease is a major threat to the grass carp aquaculture industry. Viruses are obligate intracellular parasites that require host cell machinery to complete their life cycle; the mechanism by which GCRV hijacks the host metabolism to benefit viral replication and assembly remains unclear. Our study demonstrated that GCRV infection alters host lipid metabolism and increases de novo fatty acid synthesis. The increased de novo fatty acid synthesis induced accumulation of LDs, which act as sites or scaffolds for GCRV replication and assembly. Our findings illustrate a typical example of how the virus hijacks cellular organelles for replication and assembly and hence may provide new insights for the prevention and control of GCRV.
Keywords: assembly; grass carp reovirus; lipid droplets; lipidomic analysis; replication; viroplasms.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Fisheries Bureau of Ministry of Agriculture in China. 2021. China fishery statistical yearbook of 2021. China Agriculture Press, Beijing, China.
-
- Attoui H, Mertens PPC, Becnel J, Belaganahalli S, Bergoin M, Brussaard CP, Chappell JD, Ciarlet M, del Vas M, Dermody TS, Dormitzer PR, Duncan R, Fang Q, Graham R, Guglielmi KM, Harding RM, Hillman B, Makkay A, Marzachì C, Matthijnssens J, Milne RG, Mohd Jaafar F, Mori H, Noordeloos AA, Omura T, Patton JT, Rao S, Maan M, Stoltz D, Suzuki N, Upadhyaya NM, Wei C, Zhou H. 2012. Family Reoviridae, p 477–637. In King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ (ed), Virus taxonomy. Classification and nomenclature of viruses. Ninth report of the International Committee on Taxonomy of Viruses. Elsevier Academic Press, San Diego, CA.
-
- Chu P, He L, Huang R, Liao L, Li Y, Zhu Z, Hu W, Wang Y. 2020. Autophagy inhibits grass carp reovirus (GCRV) replication and protects Ctenopharyngodon idella kidney (CIK) cells from excessive inflammatory responses after GCRV infection. Biomolecules 10:1296. doi:10.3390/biom10091296. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
