Defective Interfering Particles with Broad-Acting Antiviral Activity for Dengue, Zika, Yellow Fever, Respiratory Syncytial and SARS-CoV-2 Virus Infection
- PMID: 36445148
- PMCID: PMC9769664
- DOI: 10.1128/spectrum.03949-22
Defective Interfering Particles with Broad-Acting Antiviral Activity for Dengue, Zika, Yellow Fever, Respiratory Syncytial and SARS-CoV-2 Virus Infection
Abstract
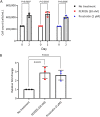
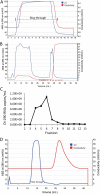
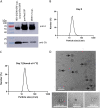
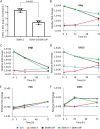
More than 100 arboviruses, almost all of which have an RNA genome, cause disease in humans. RNA viruses are causing unprecedented health system challenges worldwide, many with little or no specific therapies or vaccines available. Certain species of mosquito can carry dengue virus (DENV), Zika virus (ZIKV) and yellow fever virus (YFV), where co-infection of these viruses has occurred. Here, we found that purified synthetic defective interfering particles (DIPs) derived from DENV type 2 (DENV-2) strongly suppressed replication of the aforementioned viruses, respiratory syncytial virus (RSV) and also the novel emerging virus SARS-CoV-2 in human cells. DENV DIPs produced in bioreactors, purified by column chromatography, and concentrated are virus-like particles that are about half the diameter of a typical DENV particle, but with similar ratios of the viral structural proteins envelope and capsid. Overall, DIP-treated cells inhibited DENV, ZIKV, YFV, RSV, and SARS-CoV-2 by at least 98% by mechanisms which included interferon (IFN)-dependent cellular antiviral responses. IMPORTANCE DIPs are spontaneously derived virus mutants with deletions in genes that block viral replication. DIPs play important roles in modulation of viral disease, innate immune responses, virus persistence and virus evolution. Here, we investigated the antiviral activity of highly purified synthetic DIPs derived from DENV, which were produced in bioreactors. DENV DIPs purified by column chromatography strongly inhibited five different RNA viruses, including DENV, ZIKV, YFV, RSV, and SARS-CoV-2 in human cells. DENV DIPs inhibited virus replication via delivery of a small, noninfectious viral RNA that activated cellular innate immunity, resulting in robust type 1 interferon responses. The work here presents a pathway for DIP production which is adaptable to Good Manufacturing Practice, so that their preclinical testing should be suitable for evaluation in subjects.
Keywords: RNA; SARS-CoV-2; Zika virus; antiviral therapy; chromatography; coronavirus; defective interfering particle; defective viral genome; dengue virus; purification; respiratory syncytial virus; yellow fever virus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Broad-Spectrum Antiviral Activity of Influenza A Defective Interfering Particles against Respiratory Syncytial, Yellow Fever, and Zika Virus Replication In Vitro.Viruses. 2023 Sep 4;15(9):1872. doi: 10.3390/v15091872. Viruses. 2023. PMID: 37766278 Free PMC article.
-
Comparative Infections of Zika, Dengue, and Yellow Fever Viruses in Human Cytotrophoblast-Derived Cells Suggest a Gating Role for the Cytotrophoblast in Zika Virus Placental Invasion.Microbiol Spectr. 2023 Jun 15;11(3):e0063023. doi: 10.1128/spectrum.00630-23. Epub 2023 May 25. Microbiol Spectr. 2023. PMID: 37227282 Free PMC article.
-
Dengue virus-free defective interfering particles have potent and broad anti-dengue virus activity.Commun Biol. 2021 May 11;4(1):557. doi: 10.1038/s42003-021-02064-7. Commun Biol. 2021. PMID: 33976375 Free PMC article.
-
Immune Response to Dengue and Zika.Annu Rev Immunol. 2018 Apr 26;36:279-308. doi: 10.1146/annurev-immunol-042617-053142. Epub 2018 Jan 18. Annu Rev Immunol. 2018. PMID: 29345964 Free PMC article. Review.
-
Current status, challenges and perspectives in the development of vaccines against yellow fever, dengue, Zika and chikungunya viruses.Acta Trop. 2018 Jun;182:257-263. doi: 10.1016/j.actatropica.2018.03.009. Epub 2018 Mar 15. Acta Trop. 2018. PMID: 29551394 Review.
Cited by
-
Mathematical model calibrated to in vitro data predicts mechanisms of antiviral action of the influenza defective interfering particle "OP7".iScience. 2024 Mar 5;27(4):109421. doi: 10.1016/j.isci.2024.109421. eCollection 2024 Apr 19. iScience. 2024. PMID: 38523782 Free PMC article.
-
Harnessing defective interfering particles and lipid nanoparticles for effective delivery of an anti-dengue virus RNA therapy.Mol Ther Nucleic Acids. 2024 Dec 12;36(1):102424. doi: 10.1016/j.omtn.2024.102424. eCollection 2025 Mar 11. Mol Ther Nucleic Acids. 2024. PMID: 39817192 Free PMC article.
-
Novel and repurposed antiviral molecules for arbovirus infections with epidemic Potential: A systematic review.New Microbes New Infect. 2025 Jul 10;66:101614. doi: 10.1016/j.nmni.2025.101614. eCollection 2025 Aug. New Microbes New Infect. 2025. PMID: 40776987 Free PMC article. Review.
-
Evolution of Zika virus in Rag1-deficient mice selects for unique envelope glycosylation motif mutants that show enhanced replication fitness.Virus Evol. 2025 Apr 11;11(1):veaf021. doi: 10.1093/ve/veaf021. eCollection 2025. Virus Evol. 2025. PMID: 40291117 Free PMC article.
-
Defective viral genomes: advances in understanding their generation, function, and impact on infection outcomes.mBio. 2024 May 8;15(5):e0069224. doi: 10.1128/mbio.00692-24. Epub 2024 Apr 3. mBio. 2024. PMID: 38567955 Free PMC article. Review.
References
-
- Moquin SA, Simon O, Karuna R, Lakshminarayana SB, Yokokawa F, Wang F, Saravanan C, Zhang J, Day CW, Chan K, Wang QY, Lu S, Dong H, Wan KF, Lim SP, Liu W, Seh CC, Chen YL, Xu H, Barkan DT, Kounde CS, Sim WLS, Wang G, Yeo HQ, Zou B, Chan WL, Ding M, Song JG, Li M, Osborne C, Blasco F, Sarko C, Beer D, Bonamy GMC, Sasseville VG, Shi PY, Diagana TT, Yeung BKS, Gu F. 2021. NITD-688, a pan-serotype inhibitor of the dengue virus NS4B protein, shows favorable pharmacokinetics and efficacy in preclinical animal models. Sci Transl Med 13:eabb2181. doi:10.1126/scitranslmed.abb2181. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

