Early onset hereditary neuronopathies: an update on non-5q motor neuron diseases
- PMID: 36445400
- PMCID: PMC9976982
- DOI: 10.1093/brain/awac452
Early onset hereditary neuronopathies: an update on non-5q motor neuron diseases
Abstract
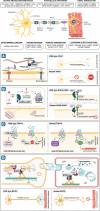
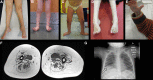
Hereditary motor neuropathies (HMN) were first defined as a group of neuromuscular disorders characterized by lower motor neuron dysfunction, slowly progressive length-dependent distal muscle weakness and atrophy, without sensory involvement. Their cumulative estimated prevalence is 2.14/100 000 and, to date, around 30 causative genes have been identified with autosomal dominant, recessive,and X-linked inheritance. Despite the advances of next generation sequencing, more than 60% of patients with HMN remain genetically uncharacterized. Of note, we are increasingly aware of the broad range of phenotypes caused by pathogenic variants in the same gene and of the considerable clinical and genetic overlap between HMN and other conditions, such as Charcot-Marie-Tooth type 2 (axonal), spinal muscular atrophy with lower extremities predominance, neurogenic arthrogryposis multiplex congenita and juvenile amyotrophic lateral sclerosis. Considering that most HMN present during childhood, in this review we primarily aim to summarize key clinical features of paediatric forms, including recent data on novel phenotypes, to help guide differential diagnosis and genetic testing. Second, we describe newly identified causative genes and molecular mechanisms, and discuss how the discovery of these is changing the paradigm through which we approach this group of conditions.
Keywords: HMN; SMA-LED; dHMN; distal SMA; paediatric neuronopathies.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures

References
-
- Harding AE, Thomas PK. Hereditary distal spinal muscular atrophy: A report on 34 cases and a review of the literature. J Neurol Sci. 1980;45:337–348. - PubMed
-
- De Jonghe P, Timmerman V, Van Broeckhoven C. 2nd Workshop of the European CMT consortium: 53rd ENMC international workshop on classification and diagnostic guidelines for Charcot-Marie-Tooth Type 2 (CMT2–HMSN II) and distal hereditary motor neuropathy (distal HMN–spinal CMT): 26–28 September 1997, Naarden, The Netherlands. Neuromuscul Disord. 1998;8:426–431. - PubMed
-
- Rossor AM, Evans MRB, Reilly MM. A practical approach to the genetic neuropathies. Pract Neurol. 2015;15:187–198. - PubMed
-
- Frasquet M, Rojas-García R, Argente-Escrig H, et al. . Distal hereditary motor neuropathies: Mutation spectrum and genotype-phenotype correlation. Eur J Neurol. 2021;28:1334–1343. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

