Neurological Complications Following COVID-19 Vaccination
- PMID: 36445631
- PMCID: PMC9707152
- DOI: 10.1007/s11910-022-01247-x
Neurological Complications Following COVID-19 Vaccination
Abstract
Purpose of review: A variety of neurological complications have been reported following the widespread use of the COVID-19 vaccines which may lead to vaccine hesitancy and serve as a major barrier to the public health aim of achieving protective herd immunity by vaccination. In this article, we review the available evidence regarding these neurological adverse events reported, to provide clarity regarding the same so that unfounded fears maybe put to rest.
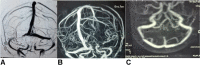
Recent findings: There is a greater than expected occurrence of severe neurological adverse events such as cortical sinus venous thrombosis, Bell's palsy, transverse myelitis, and Guillain-Barré syndromes along with other common effects such as headaches following different kinds of COVID-19 vaccination. Precipitation of new onset demyelinating brain lesions with or without detection of specific antibodies and worsening of pre-existing neurological disorders (like epilepsy, multiple sclerosis) are also a matter of great concern though no conclusive evidence implicating the vaccines is available as of now. The COVID-19 pandemic is far from being over. Till such time that a truly effective anti-viral drug is discovered, or an appropriate therapeutic strategy is developed, COVID-appropriate behavior and highly effective mass vaccination remain the only weapons in our armamentarium to fight this deadly disease. As often occurs with most therapeutic means for the treatment and prevention of any disease, vaccination against COVID-19 has its hazards. These range from the most trivial ones like fever, local pain and myalgias to several potentially serious cardiac and neurological complications. The latter group includes conditions like cerebral venous thrombosis (curiously often with thrombocytopenia), transverse myelitis and acute inflammatory demyelinating polyneuropathy amongst others. Fortunately, the number of reported patients with any of these serious complications is far too low for the total number of people vaccinated. Hence, the current evidence suggests that the benefits of vaccination far outweigh the risk of these events in majority of the patients. As of now, available evidence also does not recommend withholding vaccination in patients with pre-existing neurological disorders like epilepsy and MS, though adenoviral vaccines should be avoided in those with history of thrombotic events.
Keywords: COVID-19 vaccination; Cortical sinus venous thrombosis; Guillain–Barré syndrome; Multiple sclerosis (MS); Neurological complications; Transverse myelitis (TM).
© 2022. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
Aparajita Chatterjee and Ambar Chakravarty each declare no potential conflicts of interest.
Figures

Comment in
-
The Number of Neurological Side Effects of SARS-CoV-2 Vaccinations is Increasing.Neurol India. 2023 Jul-Aug;71(4):790-791. doi: 10.4103/0028-3886.383838. Neurol India. 2023. PMID: 37635524 No abstract available.
References
-
- World Health Organization. Global vaccine action plan 2011–2020. WHO. https://www.who.int/immunization/global_vaccine_action_plan/GVAP_doc_201... (2013). Accessed Oct 2022.
-
- World Health Organization. Child mortality and causes of death. WHO. https://www.who.int/gho/child_health/mortality/mortality_under_five_text... (2020). Accessed Oct 2022.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

