Utilizing Biologics in Drug Desensitization
- PMID: 36445652
- PMCID: PMC9707161
- DOI: 10.1007/s11882-022-01052-z
Utilizing Biologics in Drug Desensitization
Abstract
Purpose of review: The purpose of this literature review was to review the latest advancements with biologics in rapid drug desensitization. Our methodology was to highlight both desensitization to biologics themselves and the use of biologics in desensitization to both biologic and nonbiologic drugs.
Recent findings: Biologics are a vast category of drugs that include monoclonal antibodies, nanobodies, modern vaccinations, and even hormones. Desensitization to biologics can be safely performed through standardized procedure. Biomarkers are used both in vitro and in vivo to help identify and classify hypersensitivity reactions. Hypersensitivity reactions to the mRNA vaccinations against SARS-CoV-2 present their own unique challenges to management. There are specific excipients in monoclonal antibodies that are thought to be responsible for many of their hypersensitivity reactions. Certain biologics can even be used to assist in desensitization to other drugs. Rapid drug desensitization is a standardized procedure that may be able to help many patients who have experienced hypersensitivity reactions to biologics and would best be treated with them to continue to receive them. Biologic drugs have opened a new era in medicine for the prevention and treatment of infectious diseases, cancer, and inflammatory diseases. Hypersensitivity reactions to biologics are quite common. This literature review presents the latest advancements in our understanding of hypersensitivity reactions to biologics, how rapid drug desensitization can be used to continue therapy despite history of hypersensitivity, and how biologics themselves can be used to aid in desensitization itself.
Keywords: Biologics; COVID-19 vaccine; Desensitization; Hypersensitivity; Monoclonal antibodies; Nanobodies.
© 2022. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
Dr. Castells is the Anaphylaxis and Drug Allergy Section Editor for the Journal. Dr. Yang is currently employed at Ribon Therapeutics. The authors did not receive support from any organization for the submitted work. The authors have no financial or proprietary interests in any material discussed in this article.
Figures
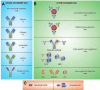

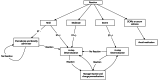


Similar articles
-
Hypersensitivity reactions to biologics in children.Expert Opin Biol Ther. 2023 Jan;23(1):61-72. doi: 10.1080/14712598.2022.2142039. Expert Opin Biol Ther. 2023. PMID: 36314361 Review.
-
Hypersensitivity reactions and anaphylaxis to checkpoint inhibitor-monoclonal antibodies and desensitization.Ann Allergy Asthma Immunol. 2021 Jun;126(6):623-629. doi: 10.1016/j.anai.2021.03.008. Epub 2021 Mar 26. Ann Allergy Asthma Immunol. 2021. PMID: 33781937 Review.
-
Hypersensitivity and immunologic reactions to biologics: opportunities for the allergist.Ann Allergy Asthma Immunol. 2016 Aug;117(2):115-20. doi: 10.1016/j.anai.2016.05.013. Ann Allergy Asthma Immunol. 2016. PMID: 27499538 Review.
-
Hypersensitivity reactions to anticancer chemotherapy and monoclonal antibodies: Safety and efficacy of desensitization.J Oncol Pharm Pract. 2024 Jul;30(5):811-822. doi: 10.1177/10781552231189461. Epub 2023 Jul 24. J Oncol Pharm Pract. 2024. PMID: 37489025
-
Rapid drug desensitization in seven patients with delayed hypersensitivity reactions to biologics and targeted therapies: Reason, successes, and failures.Allergy Asthma Proc. 2025 Mar 1;46(2):e70-e77. doi: 10.2500/aap.2025.46.240101. Allergy Asthma Proc. 2025. PMID: 40011988
Cited by
-
IQ Survey Results on Current Industry Practices: Part 2-Quantitative Evaluations of Immunogenicity Assessment.Clin Pharmacol Ther. 2025 Jun;117(6):1605-1613. doi: 10.1002/cpt.3573. Epub 2025 Feb 7. Clin Pharmacol Ther. 2025. PMID: 39921219 Free PMC article. Review.
-
Myocarditis Associated with COVID-19 Vaccination.Vaccines (Basel). 2024 Oct 19;12(10):1193. doi: 10.3390/vaccines12101193. Vaccines (Basel). 2024. PMID: 39460358 Free PMC article. Review.
-
Editorial: Diagnosis and management of allergy to chemotherapy and biologics.Front Allergy. 2023 May 11;4:1205345. doi: 10.3389/falgy.2023.1205345. eCollection 2023. Front Allergy. 2023. PMID: 37250973 Free PMC article. No abstract available.
-
Ofatumumab Desensitization Protocol: A Case of Refractory Immune Thrombocytopenic Purpura.Cureus. 2023 Sep 30;15(9):e46278. doi: 10.7759/cureus.46278. eCollection 2023 Sep. Cureus. 2023. PMID: 37908928 Free PMC article.
-
Basophil activation test is a complementary tool in the diagnosis of immediate reactions to platinum salts and taxanes.Allergy. 2025 Jan;80(1):271-286. doi: 10.1111/all.16296. Epub 2024 Aug 31. Allergy. 2025. PMID: 39215539 Free PMC article.
References
-
- Balocco R, De Sousa Guimaraes Koch S, Thorpe R, Weisser K, Malan S. New INN nomenclature for monoclonal antibodies. Lancet. 2022;399(10319):24. 10.1016/S0140-6736(21)02732-X. - PubMed
-
- WHO. New INN nomenclature scheme for monoclonal antibodies. Geneva: World Heal Org. 2022.
-
- WHO. Nomenclature for monoclonal antibodies. Geneva: World Heal Org. 2009.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

