Impact of trans-stent gradient on outcome after PCI: results from a HAWKEYE substudy
- PMID: 36445673
- PMCID: PMC9708807
- DOI: 10.1007/s10554-022-02708-7
Impact of trans-stent gradient on outcome after PCI: results from a HAWKEYE substudy
Abstract
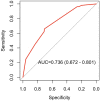
To test whether quantitative flow ratio (QFR)-based trans-stent gradient (TSG) is associated with adverse clinical events at follow-up. A post-hoc analysis of the multi-center HAWKEYE study was performed. Vessels post-PCI were divided into four groups (G) as follows: G1: QFR ≥ 0.90 TSG = 0 (n = 412, 54.8%); G2: QFR ≥ 0.90, TSG > 0 (n = 216, 28.7%); G3: QFR < 0.90, TSG = 0 (n = 37, 4.9%); G4: QFR < 0.90, TSG > 0 (n = 86, 11.4%). Cox proportional hazards regression model was used to analyze the effect of baseline and prognostic variables. The final reduced model was obtained by backward stepwise variable selection. Receiver operating characteristic (ROC) was plotted and area under the curve (AUC) was calculated and reported. Overall, 449 (59.8%) vessels had a TSG = 0 whereas (40.2%) had TSG > 0. Ten (2.2%) vessel-oriented composite endpoint (VOCE) occurred in vessels with TSG = 0, compared with 43 (14%) in vessels with TSG > 0 (p < 0.01). ROC analysis showed an AUC of 0.74 (95% CI: 0.67 to 0.80; p < 0.001). TSG > 0 was an independent predictor of the VOCE (HR 2.95 [95% CI 1.77-4.91]). The combination of higher TSG and lower final QFR (G4) showed the worst long-term outcome while low TSG and high QFR showed the best outcome (G1) while either high TSG or low QFR (G2, G3) showed intermediate and comparable outcomes. Higher trans-stent gradient was an independent predictor of adverse events and identified a subgroup of patients at higher risk for poor outcomes even when vessel QFR was optimal (> 0.90).
Keywords: Angiography-based fractional flow reserve; Outcome; Percutaneous coronary intervention; Trans-stent gradient; Vessel-oriented composite endpoint.
© 2022. The Author(s).
Conflict of interest statement
Conflict of interest: SB received research grant from Medis, SMT, Siemens, Insight Lifetech, GE and personal fees from Siemens and Insight Lifetech. GC received research grant from Boston Scientific, Medis, SMT, Siemens, Insight Lifetech. MT received research grant from Boston Scientific. BFU received research grant from Opsens.
SB received research grant from Medis, SMT, Siemens, Insight Lifetech, GE and personal fees from Siemens and Insight Lifetech. GC received research grant from Boston Scientific, Medis, SMT, Siemens, Insight Lifetech. MT received research grant from Boston.
Figures

References
-
- Pavasini R, Biscaglia S, Barbato E, et al. Complete revascularization reduces cardiovascular death in patients with ST-segment elevation myocardial infarction and multivessel disease: systematic review and meta-analysis of randomized clinical trials. Eur Heart J. 2022 doi: 10.1093/eurheartj/ehz896. - DOI - PubMed
-
- Sabaté M, Brugaletta S, Cequier A, et al. Clinical outcomes in patients with ST-segment elevation myocardial infarction treated with everolimus-eluting stents versus bare-metal stents (EXAMINATION): 5-year results of a randomised trial. The Lancet. 2016;387(10016):357–366. doi: 10.1016/S0140-6736(15)00548-6. - DOI - PubMed
-
- von Birgelen C, Zocca P, Buiten RA, et al. Thin composite wire strut, durable polymer-coated (Resolute Onyx) versus ultrathin cobalt–chromium strut, bioresorbable polymer-coated (Orsiro) drug-eluting stents in allcomers with coronary artery disease (BIONYX): an international, single-blind, randomi. The Lancet. 2018;392(10154):1235–1245. doi: 10.1016/S0140-6736(18)32001-4. - DOI - PubMed
-
- Biscaglia S, Uretsky B, Barbato E, et al. Invasive coronary physiology after stent implantation: another step toward precision medicine. Cardiovasc Interv. 2021;14(3):237–246. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

