Identification of prolactin receptor variants with diverse effects on receptor signalling
- PMID: 36445946
- PMCID: PMC7614258
- DOI: 10.1530/JME-22-0164
Identification of prolactin receptor variants with diverse effects on receptor signalling
Abstract
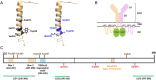
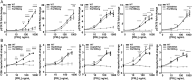
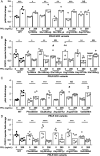
The prolactin receptor (PRLR) signals predominantly through the JAK2-STAT5 pathway regulating multiple physiological functions relating to fertility, lactation, and metabolism. However, the molecular pathology and role of PRLR mutations and signalling are incompletely defined, with progress hampered by a lack of reported disease-associated PRLR variants. To date, two common germline PRLR variants are reported to demonstrate constitutive activity, with one, Ile146Leu, overrepresented in benign breast disease, while a rare activating variant, Asn492Ile, is reported to be associated with an increased incidence of prolactinoma. In contrast, an inactivating germline heterozygous PRLR variant (His188Arg) was reported in a kindred with hyperprolactinaemia, while an inactivating compound heterozygous PRLR variant (Pro269Leu/Arg171Stop) was identified in an individual with hyperprolactinaemia and agalactia. We hypothesised that additional rare germline PRLR variants, identified from large-scale sequencing projects (ExAC and GnomAD), may be associated with altered in vitro PRLR signalling activity. We therefore evaluated >300 previously uncharacterised non-synonymous, germline PRLR variants and selected 10 variants for in vitro analysis based on protein prediction algorithms, proximity to known functional domains and structural modelling. Five variants, including extracellular and intracellular domain variants, were associated with altered responses when compared to the wild-type receptor. These altered responses included loss- and gain-of-function activities related to STAT5 signalling, Akt and FOXO1 activity, as well as cell viability and apoptosis. These studies provide further insight into PRLR structure-function and indicate that rare germline PRLR variants may have diverse modulating effects on PRLR signalling, although the pathophysiologic relevance of such alterations remains to be defined.
Keywords: JAK-STAT5; hyperprolactinaemia; prolactinoma; proliferation.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures





Similar articles
-
Association of prolactin receptor (PRLR) variants with prolactinomas.Hum Mol Genet. 2019 Mar 15;28(6):1023-1037. doi: 10.1093/hmg/ddy396. Hum Mol Genet. 2019. PMID: 30445560 Free PMC article.
-
Germline Prolactin Receptor Mutation Is Not a Major Cause of Sporadic Prolactinoma in Humans.Neuroendocrinology. 2016;103(6):738-45. doi: 10.1159/000442981. Epub 2015 Dec 8. Neuroendocrinology. 2016. PMID: 26641246
-
PRL/PRLR Can Promote Insulin Resistance by Activating the JAK2/STAT5 Signaling Pathway.Comput Math Methods Med. 2022 Oct 4;2022:1456187. doi: 10.1155/2022/1456187. eCollection 2022. Comput Math Methods Med. 2022. Retraction in: Comput Math Methods Med. 2023 Nov 29;2023:9897853. doi: 10.1155/2023/9897853. PMID: 36238467 Free PMC article. Retracted.
-
Prolactin receptor gene transcriptional control, regulatory modalities relevant to breast cancer resistance and invasiveness.Front Endocrinol (Lausanne). 2022 Sep 15;13:949396. doi: 10.3389/fendo.2022.949396. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36187116 Free PMC article. Review.
-
Prolactin receptor signaling: A novel target for cancer treatment - Exploring anti-PRLR signaling strategies.Front Endocrinol (Lausanne). 2023 Jan 13;13:1112987. doi: 10.3389/fendo.2022.1112987. eCollection 2022. Front Endocrinol (Lausanne). 2023. PMID: 36714582 Free PMC article. Review.
Cited by
-
Glycolaldehyde-derived advanced glycation end products promote macrophage proliferation via the JAK-STAT signaling pathway.Mol Biol Rep. 2023 Jul;50(7):5849-5858. doi: 10.1007/s11033-023-08509-y. Epub 2023 May 25. Mol Biol Rep. 2023. PMID: 37227674
-
Current Insights in Prolactin Signaling and Ovulatory Function.Int J Mol Sci. 2024 Feb 6;25(4):1976. doi: 10.3390/ijms25041976. Int J Mol Sci. 2024. PMID: 38396659 Free PMC article. Review.
-
c-Jun regulates postpartum β-cell apoptosis and survival downstream of prolactin signaling.Mol Cell Endocrinol. 2025 Sep 1;606:112570. doi: 10.1016/j.mce.2025.112570. Epub 2025 May 9. Mol Cell Endocrinol. 2025. PMID: 40350071
References
-
- Acosta JJ, Munoz RM, Gonzalez L, Subtil-Rodriguez A, Dominguez-Caceres MA, Garcia-Martinez JM, Calcabrini A, Lazaro-Trueba I, Martin-Perez J.2003Src mediates prolactin-dependent proliferation of T47D and MCF7 cells via the activation of focal adhesion kinase/ERK1/2 and phosphatidylinositol 3-kinase pathways. Molecular Endocrinology 172268–2282. (10.1210/me.2002-0422) - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

