Potential Benefit of Probiotic E. Coli Nissle in Term Neonates
- PMID: 36446590
- PMCID: PMC10328724
- DOI: 10.1055/a-1970-4340
Potential Benefit of Probiotic E. Coli Nissle in Term Neonates
Erratum in
-
Correction: Potential Benefit of Probiotic E. Coli Nissle in Term Neonates.Klin Padiatr. 2023 Jul;235(4):e17. doi: 10.1055/a-1991-9977. Epub 2022 Dec 13. Klin Padiatr. 2023. PMID: 36513100 Free PMC article. No abstract available.
Abstract
Background: Probiotics are often viewed as an immunity enhancing agent. The objective of this study was to investigate whether oral administration of Escherichia coli Nissle 1917 reduces the number of infections, their duration, and severity in the first 24 months after parturition in healthy neonates.
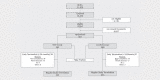
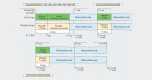
Subjects and methods: This prospective, confirmatory, randomised, double-blind, placebo-controlled study enrolled 567 healthy neonates from four German and two Polish sites. Neonates received 10e8 viable E. coli Nissle (n=283) or placebo (n=284) daily in the first week and every second day in week 2 and 3. After 6 and 12 months, the subjects received additional instillations on ten subsequent days. The overall efficacy was assessed by the number of infections per observation period.
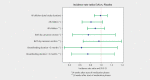
Results: Incidence rates of infection, infection duration and severity showed no statistically significant difference between groups after 24 months. Post-hoc analyses, however, revealed a short-term benefit of E. coli Nissle four weeks after treatment start which became less pronounced after eight weeks. E. coli Nissle was safe and well tolerated.
Conclusions: A long-term effect after colonising the healthy neonate´s gut with E. coli Nissle to protect against infections could not be shown. Additional studies are needed to confirm a transitory, yet clinically significant role of probiotics in the first four weeks after parturition.
Hintergrund: Probiotika werden oftmals als Immunstimulans gewertet. In dieser Studie wurde untersucht, ob sich für reife Neugeborene durch die perorale Verabreichung von Escherichia coli Nissle 1917 die Zahl der Infektionen, ihre Dauer und Schwere in den ersten 24 Monaten nach Geburt verringert.
Probanden und methoden: 567 gesunde Neugeborene aus vier deutschen und zwei polnischen Studienzentren wurden in diese prospektive, konfirmatorische, randomisierte, doppelblinde und Plazebo-kontrollierte Studie eingeschlossen. Neugeborene erhielten 10e8 lebende E. coli Nissle (n=283) oder Plazebo (n=284) täglich in der ersten Woche und jeden zweiten Tag in Woche 2 und 3. Nach 6 und 12 Monaten fanden zusätzliche Verabreichungen an zehn aufeinanderfolgenden Tagen statt. Die Wirksamkeit insgesamt wurde anhand der Zahl der Infektionen im Beobachtungszeitraum beurteilt.
Ergebnisse: Nach 24 Monaten bestand zwischen den Gruppen kein statistisch signifikanter Unterschied in den Inzidenzraten der Infektionen, Dauer oder Schwere der Infektionen. Post-hoc Analysen ergaben allerdings einen kurzfristigen Nutzen von E. coli Nissle vier Wochen nach Beginn der Therapie, der nach acht Wochen nachgelassen hatte. E. coli Nissle war sicher und wurde gut toleriert.
Schlussfolgerungen: Es konnte kein gegen Infektionen schützender Langzeiteffekt nach intestinaler Besiedlung mit E. coli Nissle für den gesundenen Neugeborenen gezeigt werden. Zusätzliche Studien sind nötig, um die vorübergehende, jedoch klinisch signifikante Rolle des Probiotikums in den ersten vier Wochen nach Entbindung zu bestätigen.
The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/).
Conflict of interest statement
D. Olbertz received an investigator’s fee and reimbursements for two related congress presentations and associated travel from Ardeypharm GmbH; C. Wolff was employed by Ardeypharm GmbH for the duration of the study; M. Radke received consultancy fees in relation to the project from Ardeypharm GmbH. All authors received reimbursement of study-related expenditures from Ardeypharm GmbH.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

