Anti-Hu Antibodies in Patients With Neurologic Side Effects of Immune Checkpoint Inhibitors
- PMID: 36446613
- PMCID: PMC9709718
- DOI: 10.1212/NXI.0000000000200058
Anti-Hu Antibodies in Patients With Neurologic Side Effects of Immune Checkpoint Inhibitors
Abstract
Background and objectives: To clinically characterize post-immune checkpoint inhibitor (ICI) Hu antibody (Ab) neurologic disorders, we analyzed Hu-Ab-positive patients with neurologic immune-related adverse events (n-irAEs) and compared them with patients with other n-irAEs, ICI-naive patients with Hu-Ab paraneoplastic neurologic syndromes (PNSs) identified in the same study center, and those with Hu-Ab n-irAEs reported elsewhere.
Methods: Patients whose samples were sent to the French reference center for a suspicion of n-irAE (2015-2021) were identified; those with a final diagnosis of n-irAE and Hu-Ab were included. Control groups included patients with a final diagnosis of n-irAE occurring during the same period as the patients included (2018-2021) but without Hu-Ab, and ICI-naive patients with Hu-Ab PNS diagnosed during the same period; a systematic review was performed to identify previous reports.
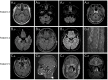
Results: Eleven patients with Hu-Ab and n-irAEs were included (median age, 66 years, range 44-76 years; 73% men). Ten patients had small cell lung cancer, and 1 had lung adenocarcinoma. The median follow-up from onset was 3 months (range 0.5-18 months). Compared with those with other n-irAEs (n = 63), Hu-Ab-positive patients had more frequently co-occurring involvement of both central and peripheral nervous systems (36% vs 8%, p = 0.02) and limbic (54% vs 14%, p < 0.01), brainstem (27% vs 5%, p = 0.02), and dorsal root ganglia (45% vs 5%, p < 0.01) involvement. The proportion of patients with severe disability (modified Rankin Scale score >3) at diagnosis was higher among Hu-Ab n-irAEs (91% vs 52%, p = 0.02). Patients with Hu-Ab had also poorer outcome (100% vs 28%, p < 0.01) and higher mortality (91% vs 46%, p < 0.01). There was no significant difference in terms of clinical features between Hu-Ab n-irAEs and ICI-naive Hu-Ab PNS (n = 92), but there was a poorer outcome (56/78, 71%, p < 0.01) and higher mortality (26%, p < 0.01) among the former. No significant difference was found between the patients reported herein and those in the literature.
Discussion: The presence of Hu-Ab identifies a subgroup of n-irAEs that consistently reproduce the phenotypes of Hu-Ab-related PNS, supporting the hypothesis of ICI triggering or unmasking PNS. As these patients show high disability and mortality, further studies are required to investigate the underlying immunopathogenic mechanisms and to improve the outcome of Hu-Ab n-irAEs.
Copyright © 2022 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
