Early Immunotherapy and Longer Corticosteroid Treatment Are Associated With Lower Risk of Relapsing Disease Course in Pediatric MOGAD
- PMID: 36446614
- PMCID: PMC9709714
- DOI: 10.1212/NXI.0000000000200065
Early Immunotherapy and Longer Corticosteroid Treatment Are Associated With Lower Risk of Relapsing Disease Course in Pediatric MOGAD
Abstract
Background and objectives: We sought to identify early factors associated with relapse and outcome in paediatric-onset myelin oligodendrocyte glycoprotein antibody-associated disorders (MOGAD).
Methods: In a multicenter retrospective cohort of pediatric MOGAD (≤18 years), onset features and treatment were compared in patients with monophasic vs relapsing disease (including cases with follow-up ≥12 months after onset or relapse at any time) and in patients with final Expanded Disability Status Scale (EDSS) 0 vs ≥1 at last follow-up (including cases with follow-up >3 months after last event or EDSS0 at any time). Multivariable logistic regression models were used to evaluate factors associated with relapsing disease course and EDSS ≥ 1 at final follow-up.
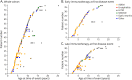
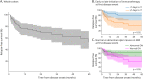
Results: Seventy-five children were included (median onset age 7 years; median 30 months of follow-up). Presentation with acute disseminated encephalomyelitis was more frequent in children aged 8 years or younger (66.7%, 28/42) than in older patients (30.3%, 10/33) (p = 0.002), whereas presentation with optic neuritis was more common in children older than 8 years (57.6%, 19/33) than in younger patients (21.4%, 9/42) (p = 0.001). 40.0% (26/65) of patients relapsed. Time to first relapse was longer in children aged 8 years or younger than in older patients (median 18 vs 4 months) (p = 0.013). Factors at first event independently associated with lower risk of relapsing disease course were immunotherapy <7 days from onset (6.7-fold reduced odds of relapsing course, OR 0.15, 95% CI 0.03-0.61, p = 0.009), corticosteroid treatment for ≥5 weeks (6.7-fold reduced odds of relapse, OR 0.15, 95% CI 0.03-0.80, p = 0.026), and abnormal optic nerves on onset MRI (12.5-fold reduced odds of relapse, OR 0.08, 95% CI 0.01-0.50, p = 0.007). 21.1% (15/71) had EDSS ≥ 1 at final follow-up. Patients with a relapsing course had a higher proportion of final EDSS ≥ 1 (37.5%, 9/24) than children with monophasic disease (12.8%, 5/39) (p = 0.022, univariate analysis). Each 1-point increment in worst EDSS at onset was independently associated with 6.7-fold increased odds of final EDSS ≥ 1 (OR 6.65, 95% CI 1.33-33.26, p = 0.021).
Discussion: At first attack of pediatric MOGAD, early immunotherapy, longer duration of corticosteroid treatment, and abnormal optic nerves on MRI seem associated with lower risk of relapse, whereas higher disease severity is associated with greater risk of final disability (EDSS ≥ 1).
Copyright © 2022 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures
References
-
- Baumann M, Hennes EM, Schanda K, et al. . Children with multiphasic disseminated encephalomyelitis and antibodies to the myelin oligodendrocyte glycoprotein (MOG): extending the spectrum of MOG antibody positive diseases. Mult Scler. 2016;22(14):1821-1829. doi: 10.1177/1352458516631038 - DOI - PubMed
-
- Bruijstens AL, Lechner C, Flet-Berliac L, et al. . E.U. paediatric MOG consortium consensus: Part 1—classification of clinical phenotypes of paediatric myelin oligodendrocyte glycoprotein antibody-associated disorders. Eur J Paediatr Neurol. 2020;29:2-13. doi: 10.1016/j.ejpn.2020.10.006 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
