Evaluation of immune response to single dose of quadrivalent HPV vaccine at 10-year post-vaccination
- PMID: 36446654
- PMCID: PMC9792650
- DOI: 10.1016/j.vaccine.2022.11.044
Evaluation of immune response to single dose of quadrivalent HPV vaccine at 10-year post-vaccination
Abstract
Background: The recent World Health Organization recommendation supporting single-dose of HPV vaccine will significantly reduce programmatic cost, mitigate the supply shortage, and simplify logistics, thus allowing more low- and middle-income countries to introduce the vaccine. From a programmatic perspective the durability of protection offered by a single-dose will be a key consideration. The primary objectives of the present study were to determine whether recipients of a single-dose of quadrivalent HPV vaccine had sustained immune response against targeted HPV types (HPV 6,11,16,18) at 10 years post-vaccination and whether this response was superior to the natural antibody titres observed in unvaccinated women.
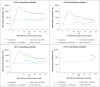
Methods: Participants received at age 10-18 years either one, two or three doses of the quadrivalent HPV vaccine. Serology samples were obtained at different timepoints up to 10 years after vaccination from a convenience sample of vaccinated participants and from age-matched unvaccinated women at one timepoint. The evolution of the binding and neutralizing antibody response was presented by dose received. 10-year durability of immune responses induced by a single-dose was compared to that after three doses of the vaccine and in unvaccinated married women.
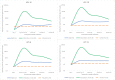
Results: The dynamics of antibody response among the single-dose recipients observed over 120 months show stabilized levels 18 months after vaccination for all four HPV types. Although the HPV type-specific (binding or neutralizing) antibody titres after a single-dose were significantly inferior to those after three doses of the vaccine (lower bounds of GMT ratios < 0.5), they were all significantly higher than those observed in unvaccinated women following natural infections (GMT ratios: 2.05 to 4.04-fold higher). The results correlate well with the high vaccine efficacy of single-dose against persistent HPV 16/18 infections reported by us earlier at 10-years post-vaccination.
Conclusion: Our study demonstrates the high and durable immune response in single-dose recipients of HPV vaccine at 10-years post vaccination.
Keywords: Human papillomavirus; Natural immunity; Single dose; Vaccine immune responses; quadrivalent HPV vaccine.
Copyright © 2022 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Gallagher K.E., LaMontagne D.S., Watson-Jones D. Status of HPV vaccine introduction and barriers to country uptake. Vaccine. 2018;36(32):4761–4767. - PubMed
-
- WHO. One-dose Human Papillomavirus (HPV) vaccine offers solid protection against cervical cancer. Available from: https://www.who.int/news/item/11-04-2022-one-dose-human-papillomavirus-(.... - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

