Chronic lymphocytic leukemia treatment algorithm 2022
- PMID: 36446777
- PMCID: PMC9708674
- DOI: 10.1038/s41408-022-00756-9
Chronic lymphocytic leukemia treatment algorithm 2022
Erratum in
-
Correction: Chronic lymphocytic leukemia treatment algorithm 2022.Blood Cancer J. 2022 Dec 22;12(12):172. doi: 10.1038/s41408-022-00775-6. Blood Cancer J. 2022. PMID: 36543762 Free PMC article. No abstract available.
Abstract
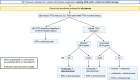
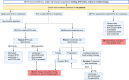
The treatment landscape for patients with chronic lymphocytic leukemia (CLL) has changed considerably with the introduction of very effective oral targeted therapies (such as Bruton tyrosine kinase inhibitors and venetoclax) and next-generation anti-CD20 monoclonal antibodies (such as obinutuzumab). These agents lead to improved outcomes in patients with CLL, even among those with high-risk features, such as del17p13 or TP53 mutation and unmutated immunoglobulin heavy chain (IGHV) genes. Selecting the right treatment for the right patient requires consideration of disease characteristics and prior treatment sequence, as well as patient preferences and comorbidities. The CLL-International Prognostic Index (CLL-IPI) remains the best-validated tool in predicting the time to first therapy among previously untreated patients, which guides selection for early intervention efforts. This review summarizes our current approach to the management of CLL, right from the time of diagnosis through relapsed disease.
© 2022. The Author(s).
Conflict of interest statement
Research funding has been provided to the institution from Janssen, AstraZeneca, Merck, and Genentech for clinical studies in which Sameer A. Parikh is a principal investigator. Honoraria has been provided to the institution from Pharmacyclics, Merck, AstraZeneca, Genentech, Amgen, TG Therapeutics, Novalgen Limited, and AbbVie for Sameer A. Parikh’s participation in consulting activities/advisory board meetings.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

