Human visual consciousness involves large scale cortical and subcortical networks independent of task report and eye movement activity
- PMID: 36446792
- PMCID: PMC9707162
- DOI: 10.1038/s41467-022-35117-4
Human visual consciousness involves large scale cortical and subcortical networks independent of task report and eye movement activity
Abstract
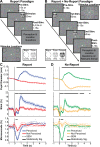
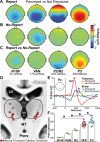
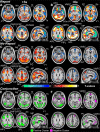
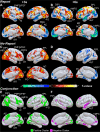
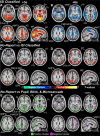
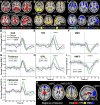
The full neural circuits of conscious perception remain unknown. Using a visual perception task, we directly recorded a subcortical thalamic awareness potential (TAP). We also developed a unique paradigm to classify perceived versus not perceived stimuli using eye measurements to remove confounding signals related to reporting on conscious experiences. Using fMRI, we discovered three major brain networks driving conscious visual perception independent of report: first, increases in signal detection regions in visual, fusiform cortex, and frontal eye fields; and in arousal/salience networks involving midbrain, thalamus, nucleus accumbens, anterior cingulate, and anterior insula; second, increases in frontoparietal attention and executive control networks and in the cerebellum; finally, decreases in the default mode network. These results were largely maintained after excluding eye movement-based fMRI changes. Our findings provide evidence that the neurophysiology of consciousness is complex even without overt report, involving multiple cortical and subcortical networks overlapping in space and time.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

