Ultrafast laser surgery probe for sub-surface ablation to enable biomaterial injection in vocal folds
- PMID: 36446830
- PMCID: PMC9708667
- DOI: 10.1038/s41598-022-24446-5
Ultrafast laser surgery probe for sub-surface ablation to enable biomaterial injection in vocal folds
Abstract
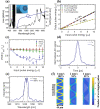
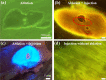
Creation of sub-epithelial voids within scarred vocal folds via ultrafast laser ablation may help in localization of injectable therapeutic biomaterials towards an improved treatment for vocal fold scarring. Several ultrafast laser surgery probes have been developed for precise ablation of surface tissues; however, these probes lack the tight beam focusing required for sub-surface ablation in highly scattering tissues such as vocal folds. Here, we present a miniaturized ultrafast laser surgery probe designed to perform sub-epithelial ablation in vocal folds. The requirement of high numerical aperture for sub-surface ablation, in addition to the small form factor and side-firing architecture required for clinical use, made for a challenging optical design. An Inhibited Coupling guiding Kagome hollow core photonic crystal fiber delivered micro-Joule level ultrashort pulses from a high repetition rate fiber laser towards a custom-built miniaturized objective, producing a 1/e2 focal beam radius of 1.12 ± 0.10 μm and covering a 46 × 46 μm2 scan area. The probe could deliver up to 3.8 μJ pulses to the tissue surface at 40% transmission efficiency through the entire system, providing significantly higher fluences at the focal plane than were required for sub-epithelial ablation. To assess surgical performance, we performed ablation studies on freshly excised porcine hemi-larynges and found that large area sub-epithelial voids could be created within vocal folds by mechanically translating the probe tip across the tissue surface using external stages. Finally, injection of a model biomaterial into a 1 × 2 mm2 void created 114 ± 30 μm beneath the vocal fold epithelium surface indicated improved localization when compared to direct injection into the tissue without a void, suggesting that our probe may be useful for pre-clinical evaluation of injectable therapeutic biomaterials for vocal fold scarring therapy. With future developments, the surgical system presented here may enable treatment of vocal fold scarring in a clinical setting.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Benninger MS, et al. Vocal fold scarring: Current concepts and management. Otolaryngol. Head. Neck Surg. 1996;115:474–482. - PubMed
-
- Friedrich, G. et al. Vocal fold scars: current concepts and future directions. Consensus report of the Phonosurgery Committee of the European Laryngological Society. Eur. Arch. oto-rhino-laryngology Off. J. Eur. Fed. Oto-Rhino-Laryngological Soc. Affil. with Ger. Soc. Oto-Rhino-Laryngologyṣ—Head Neck Surg. 270, 2491–2507 (2013). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

