Vicia faba peel extracts bearing fatty acids moieties as a cost-effective and green corrosion inhibitor for mild steel in marine water: computational and electrochemical studies
- PMID: 36446843
- PMCID: PMC9708655
- DOI: 10.1038/s41598-022-24793-3
Vicia faba peel extracts bearing fatty acids moieties as a cost-effective and green corrosion inhibitor for mild steel in marine water: computational and electrochemical studies
Abstract
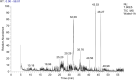
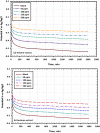
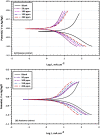
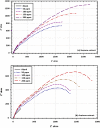
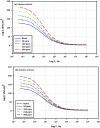
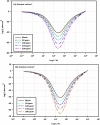
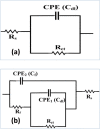
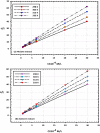
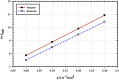
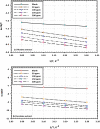
The goal of this research is to determine what chemicals are present in two different extracts (hexane and acetone) of Vicia faba (family Fabaceae, VF) peels and evaluate their effectiveness as a corrosion inhibitor on mild steel in a saline media containing 3.5% sodium chloride. Gas chromatography-mass spectrometry (GC/MS) was used to determine the composition of various extracts. It was determined that fourteen different chemicals were present in the hexane extract, the most prominent of which were octacosane, tetrasodium tetracontane, palmitic acid, and ethyl palmitate. Heptacosane, lauric acid, myristic acid, ethyl palmitate, and methyl stearate were some of the 13 chemicals found in the acetone extract. Using open circuit potential, potentiodynamic polarisation, and electrochemical impedance spectroscopic techniques, we can approximate the inhibitory effects of (VF) extracts on mild steel. The most effective inhibitory concentrations were found to be 200 ppm for both the hexane and acetone extracts (97.84% for the hexane extract and 88.67% for the acetone extract). Evaluation experiments were conducted at 298 K, with a 3.5% (wt/v) NaCl content and a flow velocity of about 250 rpm. Langmuir adsorption isotherm shows that the two extracts function as a mixed-type inhibitor in nature. Docking models were used to investigate the putative mechanism of corrosion inhibition, and GC/MS was used to identify the major and secondary components of the two extracts. Surface roughness values were calculated after analyzing the morphology of the metal's surface with and without (VF) using a scanning electron microscope (SEM). The results showed that throughout the surface of the mild steel, a thick adsorbate layer was formed. Quantum chemical calculations conducted on the two extracts as part of the theoretical research of quantum chemical calculation demonstrated a connection between the experimental analysis results and the theoretical study of the major chemical components.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures







References
-
- Allam AE, Nafady AM, Khedr AIM, Nakagawa T, Shimizu K. Potential activities for constituents from Vicia faba L. Trends Phytochem. Res. 2018;2(1):21–26.
-
- Mann, J., Davidson, R.S., Hobbs, J.B., Banthorpe, D.V., Harbourne, J.B. Natural Products, Their Chemistry and Biological Significance, 1st edn, 1–5. (Longman Scientific and Technical Longman Group, 1994).
-
- Boukhanouf S, Louaileche H, Perrin D. Phytochemical content and in vitro antioxidant activity of faba bean (Vicia faba L.) as affected by maturity stage and cooking practice. Int. Food Res. J. 2016;23(3):954–961.
-
- Duc G, Bao S, Baum M, Redden B, Sadiki M, Suso MJ, Vishniakova M, Zong X. Diversity maintenance and use of Vicia faba L. genetic resources. Field Crops Res. 2010;115:270–278. doi: 10.1016/j.fcr.2008.10.003. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

