The Genetically Informed Neurobiology of Addiction (GINA) model
- PMID: 36446900
- PMCID: PMC10041646
- DOI: 10.1038/s41583-022-00656-8
The Genetically Informed Neurobiology of Addiction (GINA) model
Abstract
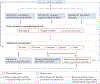
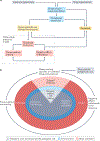
Addictions are heritable and unfold dynamically across the lifespan. One prominent neurobiological theory proposes that substance-induced changes in neural circuitry promote the progression of addiction. Genome-wide association studies have begun to characterize the polygenic architecture undergirding addiction liability and revealed that genetic loci associated with risk can be divided into those associated with a general broad-spectrum liability to addiction and those associated with drug-specific addiction risk. In this Perspective, we integrate these genomic findings with our current understanding of the neurobiology of addiction to propose a new Genetically Informed Neurobiology of Addiction (GINA) model.
© 2022. Springer Nature Limited.
Conflict of interest statement
Competing interests
The authors declare no competing interests
Figures


References
-
- Peacock A et al. Global statistics on alcohol, tobacco and illicit drug use: 2017 status report. Addiction 113, 1905–1926 (2018). - PubMed

