The human ion channel TRPM2 modulates migration and invasion in neuroblastoma through regulation of integrin expression
- PMID: 36446940
- PMCID: PMC9709080
- DOI: 10.1038/s41598-022-25138-w
The human ion channel TRPM2 modulates migration and invasion in neuroblastoma through regulation of integrin expression
Abstract
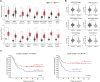
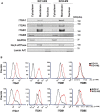
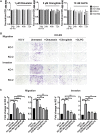
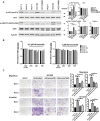
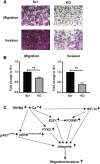
Transient receptor potential channel TRPM2 is highly expressed in many cancers and involved in regulation of key physiological processes including mitochondrial function, bioenergetics, and oxidative stress. In Stage 4 non-MYCN amplified neuroblastoma patients, high TRPM2 expression is associated with worse outcome. Here, neuroblastoma cells with high TRPM2 expression demonstrated increased migration and invasion capability. RNA sequencing, RT-qPCR, and Western blotting demonstrated that the mechanism involved significantly greater expression of integrins α1, αv, β1, and β5 in cells with high TRPM2 expression. Transcription factors HIF-1α, E2F1, and FOXM1, which bind promoter/enhancer regions of these integrins, were increased in cells with high TRPM2 expression. Subcellular fractionation confirmed high levels of α1, αv, and β1 membrane localization and co-immunoprecipitation confirmed the presence of α1β1, αvβ1, and αvβ5 complexes. Inhibitors of α1β1, αvβ1, and αvβ5 complexes significantly reduced migration and invasion in cells highly expressing TRPM2, confirming their functional role. Increased pAktSer473 and pERKThr202/Tyr204, which promote migration through mechanisms including integrin activation, were found in cells highly expressing TRPM2. TRPM2 promotes migration and invasion in neuroblastoma cells with high TRPM2 expression through modulation of integrins together with enhancing cell survival, negatively affecting patient outcome and providing rationale for TRPM2 inhibition in anti-neoplastic therapy.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Prawitt D, et al. Identification and characterization of MTR1, a novel gene with homology to melastatin (MLSN1) and the trp gene family located in the BWS-WT2 critical region on chromosome 11p15.5 and showing allele-specific expression. Hum. Mol. Genet. 2000;9:203–216. doi: 10.1093/hmg/9.2.203. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

