Mitochondria and cell death-associated inflammation
- PMID: 36447047
- PMCID: PMC9950460
- DOI: 10.1038/s41418-022-01094-w
Mitochondria and cell death-associated inflammation
Abstract
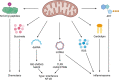
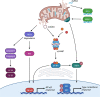
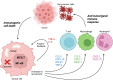
Mitochondria have recently emerged as key drivers of inflammation associated with cell death. Many of the pro-inflammatory pathways activated during cell death occur upon mitochondrial outer membrane permeabilization (MOMP), the pivotal commitment point to cell death during mitochondrial apoptosis. Permeabilised mitochondria trigger inflammation, in part, through the release of mitochondrial-derived damage-associated molecular patterns (DAMPs). Caspases, while dispensable for cell death during mitochondrial apoptosis, inhibit activation of pro-inflammatory pathways after MOMP. Some of these mitochondrial-activated inflammatory pathways can be traced back to the bacterial ancestry of mitochondria. For instance, mtDNA and bacterial DNA are highly similar thereby activating similar cell autonomous immune signalling pathways. The bacterial origin of mitochondria suggests that inflammatory pathways found in cytosol-invading bacteria may be relevant to mitochondrial-driven inflammation after MOMP. In this review, we discuss how mitochondria can initiate inflammation during cell death highlighting parallels with bacterial activation of inflammation. Moreover, we discuss the roles of mitochondrial inflammation during cell death and how these processes may potentially be harnessed therapeutically, for instance to improve cancer treatment.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

