A glyco-engineering approach for site-specific conjugation to Fab glycans
- PMID: 36447399
- PMCID: PMC9715014
- DOI: 10.1080/19420862.2022.2149057
A glyco-engineering approach for site-specific conjugation to Fab glycans
Abstract
Effective processes for synthesizing antibody-drug conjugates (ADCs) require: 1) site-specific incorporation of the payload to avoid interference with binding to the target epitope, 2) optimal drug/antibody ratio to achieve sufficient potency while avoiding aggregation or solubility problems, and 3) a homogeneous product to facilitate approval by regulatory agencies. In conventional ADCs, the drug molecules are chemically attached randomly to antibody surface residues (typically Lys or Cys), which can interfere with epitope binding and targeting, and lead to overall product heterogeneity, long-term colloidal instability and unfavorable pharmacokinetics. Here, we present a more controlled process for generating ADCs where drug is specifically conjugated to only Fab N-linked glycans in a narrow ratio range through functionalized sialic acids. Using a bacterial sialytransferase, we incorporated N-azidoacetylneuraminic acid (Neu5NAz) into the Fab glycan of cetuximab. Since only about 20% of human IgG1 have a Fab glycan, we extended the application of this approach by using molecular modeling to introduce N-glycosylation sites in the Fab constant region of other therapeutic monoclonal antibodies. We used trastuzumab as a model for the incorporation of Neu5NAz in the novel Fab glycans that we designed. ADCs were generated by clicking the incorporated Neu5NAz with monomethyl auristatin E (MMAE) attached to a self-immolative linker terminated with dibenzocyclooctyne (DBCO). Through this process, we obtained cetuximab-MMAE and trastuzumab-MMAE with drug/antibody ratios in the range of 1.3 to 2.5. We confirmed that these ADCs still bind their targets efficiently and are as potent in cytotoxicity assays as control ADCs obtained by standard conjugation protocols. The site-directed conjugation to Fab glycans has the additional benefit of avoiding potential interference with effector functions that depend on Fc glycan structure.
Keywords: Actinobacillus; Fab glycan; antibody; cetuximab; drug conjugate; sialyltransferase; trastuzumab.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures

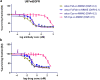
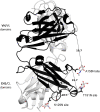
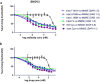
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
