A long-term study on structural changes in calcium aluminate silicate hydrates
- PMID: 36447990
- PMCID: PMC9700620
- DOI: 10.1617/s11527-022-02080-x
A long-term study on structural changes in calcium aluminate silicate hydrates
Abstract
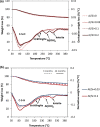
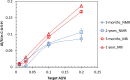
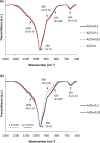
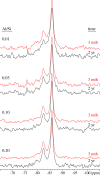
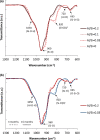
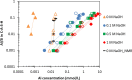
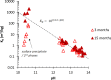
Production of blended cements in which Portland cement is combined with supplementary cementitious materials (SCM) is an effective strategy for reducing the CO2 emissions during cement manufacturing and achieving sustainable concrete production. However, the high Al2O3 and SiO2 contents of SCM change the chemical composition of the main hydration product, calcium aluminate silicate hydrate (C-A-S-H). Herein, spectroscopic and structural data for C-A-S-H gels are reported in a large range of equilibration times from 3 months up to 2 years and Al/Si molar ratios from 0.001 to 0.2. The 27Al MAS NMR spectroscopy and thermogravimetric analysis indicate that in addition to the C-A-S-H phase, secondary phases such as strätlingite, katoite, Al(OH)3 and calcium aluminate hydrate are present at Al/Si ≥ 0.03 limiting the uptake of Al in C-A-S-H. More secondary phases are present at higher Al concentrations; their content decreases with equilibration time while more Al is taken up in the C-A-S-H phase. At low Al contents, Al concentrations decrease strongly with time indicating a slow equilibration, in contrast to high Al contents where a clear change in Al concentrations over time was not observed indicating that the equilibrium has been reached faster. The 27Al NMR studies show that tetrahedrally coordinated Al is incorporated in C-A-S-H and its amount increases with the amount of Al present in the solution.
Supplementary information: The online version contains supplementary material available at 10.1617/s11527-022-02080-x.
Keywords: Aluminum; Blended cement; CO2 emission; C–A–S–H; Equilibration time; NMR.
© The Author(s) 2022.
Conflict of interest statement
Conflict of interestThe authors have no competing interests to declare that are relevant to the content of this article.
Figures






References
-
- Glavind M (2009) Sustainability of cement, concrete and cement replacement materials in construction In: Sustainability of Construction Materials. Woodhead publishing, Cambridge, UK. 10.1533/9781845695842.120
-
- Saleh HM, Eskander SB (2020) Innovative cement-based materials for environmental protection and restoration. In: Samui P, Kim D, Iyer NR, Chaudhary S (eds) New materials in civil engineering, Butterworth-Heinemann, UK, pp 613–641
-
- Thomas M, Barcelo L, Blair B, et al. Lowering the carbon footprint of concrete by reducing clinker content of cement. Transp Res Rec. 2012 doi: 10.3141/2290-13. - DOI
-
- Gartner E. Industrially interesting approaches to “low-CO2” cements. Cem Concr Res. 2004;34:1489–1498. doi: 10.1016/j.cemconres.2004.01.021. - DOI
LinkOut - more resources
Full Text Sources
