Next-generation immunotherapy for solid tumors: combination immunotherapy with crosstalk blockade of TGFβ and PD-1/PD-L1
- PMID: 36448335
- PMCID: PMC10085570
- DOI: 10.1080/13543784.2022.2152323
Next-generation immunotherapy for solid tumors: combination immunotherapy with crosstalk blockade of TGFβ and PD-1/PD-L1
Abstract
Introduction: In solid tumor immunotherapy, less than 20% of patients respond to anti-programmed cell death 1 (PD-1)/programmed cell death 1 ligand 1 (PD-L1) agents. The role of transforming growth factor β (TGFβ) in diverse immunity is well-established; however, systemic blockade of TGFβ is associated with toxicity. Accumulating evidence suggests the role of crosstalk between TGFβ and PD-1/PD-L1 pathways.
Areas covered: We focus on TGFβ and PD-1/PD-L1 signaling pathway crosstalk and the determinant role of TGFβ in the resistance of immune checkpoint blockade. We provide the rationale for combination anti-TGFβ and anti-PD-1/PD-L1 therapies for solid tumors and discuss the current status of dual blockade therapy in preclinical and clinical studies.
Expert opinion: The heterogeneity of tumor microenvironment across solid tumors complicates patient selection, treatment regimens, and response and toxicity assessment for investigation of dual blockade agents. However, clinical knowledge from single-agent studies provides infrastructure to translate dual blockade therapies. Dual TGFβ and PD-1/PD-L1 blockade results in enhanced T-cell infiltration into tumors, a primary requisite for successful immunotherapy. A bifunctional fusion protein specifically targets TGFβ in the tumor microenvironment, avoiding systemic toxicity, and prevents interaction of PD-1+ cytotoxic cells with PD-L1+ tumor cells.
Keywords: Crosstalk; PD-1; PD-L1; TGFβ; dual blockade therapy.
Conflict of interest statement
PS Adusumilli declares research funding from ATARA Biotherapeutics; Scientific Advisory Board Member and Consultant for ATARA Biotherapeutics, Bayer, Carisma Therapeutics, Imugene, ImmPactBio, Johnston & Johnston, OutpaceBio; Patents, royalties and intellectual property on mesothelin-targeted CAR and other T-cell therapies, which have been licensed to ATARA Biotherapeutics, issued patent method for detection of cancer cells using virus, and pending patent applications on PD-1 dominant negative receptor, wireless pulse-oximetry device, and on an ex vivo malignant pleural effusion culture system.
Memorial Sloan Kettering Cancer Center (MSK) has licensed intellectual property related to mesothelin-targeted CARs and T-cell therapies to ATARA Biotherapeutics and has associated financial interests.
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Figures
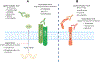



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
