Early-life fecal metabolomics of food allergy
- PMID: 36448508
- PMCID: PMC10590492
- DOI: 10.1111/all.15602
Early-life fecal metabolomics of food allergy
Abstract
Background: Intestinal microenvironmental perturbations may increase food allergy risk. We hypothesize that children with clinical food allergy, those with food sensitization, and healthy children can be differentiated by intestinal metabolites in the first years of life.
Methods: In this ancillary analysis of the Vitamin D Antenatal Asthma Reduction Trial (VDAART), we performed untargeted metabolomic profiling in 824 stool samples collected at ages 3-6 months, 1 year and 3 years. Subjects included 23 with clinical food allergy at age 3 and/or 6 years, 151 with food sensitization but no clinical food allergy, and 220 controls. We identified modules of correlated, functionally related metabolites and sought associations of metabolite modules and individual metabolites with food allergy/sensitization using regression models.
Results: Several modules of functionally related intestinal metabolites were reduced among subjects with food allergy, including bile acids at ages 3-6 months and 1 year, amino acids at age 3-6 months, steroid hormones at 1 year, and sphingolipids at age 3 years. One module primarily containing diacylglycerols was increased in those with food allergy at age 3-6 months. Fecal caffeine metabolites at age 3-6 months, likely derived from breast milk, were increased in those with food allergy and/or sensitization (beta = 5.9, 95% CI 1.0-10.8, p = .02) and were inversely correlated with fecal bile acids and bilirubin metabolites, though maternal plasma caffeine levels were not associated with food allergy and/or sensitization.
Conclusions: Several classes of bioactive fecal metabolites are associated with food allergy and/or sensitization including bile acids, steroid hormones, sphingolipids, and caffeine metabolites.
Keywords: bile acids; caffeine; food allergy; food sensitization; metabolomics.
© 2022 European Academy of Allergy and Clinical Immunology and John Wiley & Sons Ltd.
Conflict of interest statement
AAL has received author royalties from UpToDate, Inc. STW has received royalties from UpToDate, Inc and has invested in Histolix. LBB reports grants from NIH/NIAID and NHLBI, personal fees from GlaxoSmithKline Genentech/Novartis, DBV Technologies, Teva, Boehringer Ingelheim, AstraZeneca, WebMD/Medscape, Sanofi, Regeneron, Vectura, Circassia, Kinaset, Vertex, OM Pharma; and royalties from Elsevier outside the submitted work. AB holds stock from DBV Technologies, is a consultant for AstraZeneca and Raffa, and received speaking honoraria from AstraZenica, Novartis and Sanofi. RSZ is a consultant for AstraZeneca, DBV Technologies, Genentech, Inc., GlaxoSmithKline, Merck & Co., Novartis, Quest Diagnostics, Regeneron/Sanofi, TEVA Pharmaceuticals, and has received research support from ALK Pharmaceuticals, AstraZeneca, Genentech, Inc., GlaxoSmithKline, NHLBI, MedImmune, Merck, and Teva Pharmaceuticals. GTO has been compensated for speaking at a conference supported by Menarini, Inc. and for serving on a Data and Safety Monitoring Committee for Dicerna, Inc. JL-S is a Scientific Advisor for Precion. KL-S, YCC, RSK, XJ, NL, SB, PJB and DRG have nothing to disclose.
Figures
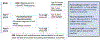


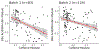
References
-
- Gupta RS, Warren CM, Smith BM, Blumenstock JA, Jiang J, Davis MM, Nadeau KC. The Public Health Impact of Parent-Reported Childhood Food Allergies in the United States. Pediatrics. 2018. Dec;142(6):e20181235. doi: 10.1542/peds.2018-1235. Epub 2018 Nov 19. Erratum in: Pediatrics. 2019 Mar;143(3) - DOI - PMC - PubMed
-
- Zierer J, Jackson MA, Kastenmüller G, Mangino M, Long T, Telenti A, Mohney RP, Small KS, Bell JT, Steves CJ, Valdes AM, Spector TD, Menni C. The fecal metabolome as a functional readout of the gut microbiome. Nat Genet. 2018. Jun;50(6):790–795. doi: 10.1038/s41588-018-0135-7. Epub 2018 May 28. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL123915/HL/NHLBI NIH HHS/United States
- R01 HL141826/HL/NHLBI NIH HHS/United States
- R01 HL108818/HL/NHLBI NIH HHS/United States
- U19 AI095219/AI/NIAID NIH HHS/United States
- K08 HL148178/HL/NHLBI NIH HHS/United States
- R01 AI143855/AI/NIAID NIH HHS/United States
- UG3 OD023268/OD/NIH HHS/United States
- T32 HL007427/HL/NHLBI NIH HHS/United States
- U01 HL091528/HL/NHLBI NIH HHS/United States
- R01 AI147028/AI/NIAID NIH HHS/United States
- T32 AI007306/AI/NIAID NIH HHS/United States
- P30 ES001247/ES/NIEHS NIH HHS/United States
- UH3 OD023268/OD/NIH HHS/United States
- K01 HL146980/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

