Loss of WNT4 in the gubernaculum causes unilateral cryptorchidism and fertility defects
- PMID: 36448532
- PMCID: PMC10112923
- DOI: 10.1242/dev.201093
Loss of WNT4 in the gubernaculum causes unilateral cryptorchidism and fertility defects
Abstract
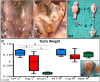
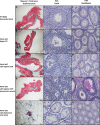
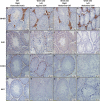
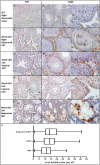
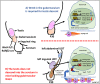
Undescended testis (UDT) affects 6% of male births. Despite surgical correction, some men with unilateral UDT may experience infertility with the contralateral descended testis (CDT) showing no A-dark spermatogonia. To improve our understanding of the etiology of infertility in UDT, we generated a novel murine model of left unilateral UDT. Gubernaculum-specific Wnt4 knockout (KO) mice (Wnt4-cKO) were generated using retinoic acid receptor β2-cre mice and were found to have a smaller left-unilateral UDT. Wnt4-cKO mice with abdominal UDT had an increase in serum follicle-stimulating hormone and luteinizing hormone and an absence of germ cells in the undescended testicle. Wnt4-cKO mice with inguinal UDT had normal hormonal profiles, and 50% of these mice had no sperm in the left epididymis. Wnt4-cKO mice had fertility defects and produced 52% fewer litters and 78% fewer pups than control mice. Wnt4-cKO testes demonstrated increased expression of estrogen receptor α and SOX9, upregulation of female gonadal genes, and a decrease in male gonadal genes in both CDT and UDT. Several WNT4 variants were identified in boys with UDT. The presence of UDT and fertility defects in Wnt4-cKO mice highlights the crucial role of WNT4 in testicular development.
Keywords: Cryptorchidism; Gubernaculum; Infertility; SOX9; Undescended testis; WNT4.
© 2022. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The Department of Molecular and Human Genetics at Baylor College of Medicine derives revenue from clinical genetic testing offered through Baylor Genetics Laboratories.
Figures





References
-
- Bainbridge, M. N., Wang, M., Wu, Y., Newsham, I., Muzny, D. M., Jefferies, J. L., Albert, T. J., Burgess, D. L. and Gibbs, R. A. (2011). Targeted enrichment beyond the consensus coding DNA sequence exome reveals exons with higher variant densities. Genome Biol. 12, R68. 10.1186/gb-2011-12-7-r68 - DOI - PMC - PubMed
-
- Barbotin, A.-L., Dauvergne, A., Dumont, A., Ramdane, N., Mitchell, V., Rigot, J.-M., Boitrelle, F. and Robin, G. (2019). Bilateral versus unilateral cryptorchidism in nonobstructive azoospermia: testicular sperm extraction outcomes. Asian J. Androl. 21, 445-451. 10.4103/aja.aja_2_19 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

