Development and Characterization of Bioinspired Lipid Raft Nanovesicles for Therapeutic Applications
- PMID: 36448709
- PMCID: PMC9756296
- DOI: 10.1021/acsami.2c13868
Development and Characterization of Bioinspired Lipid Raft Nanovesicles for Therapeutic Applications
Abstract
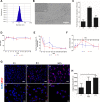
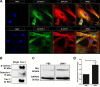
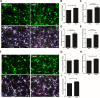
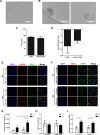
Lipid rafts are highly ordered regions of the plasma membrane enriched in signaling proteins and lipids. Their biological potential is realized in exosomes, a subclass of extracellular vesicles (EVs) that originate from the lipid raft domains. Previous studies have shown that EVs derived from human placental mesenchymal stromal cells (PMSCs) possess strong neuroprotective and angiogenic properties. However, clinical translation of EVs is challenged by very low, impure, and heterogeneous yields. Therefore, in this study, lipid rafts are validated as a functional biomaterial that can recapitulate the exosomal membrane and then be synthesized into biomimetic nanovesicles. Lipidomic and proteomic analyses show that lipid raft isolates retain functional lipids and proteins comparable to PMSC-EV membranes. PMSC-derived lipid raft nanovesicles (LRNVs) are then synthesized at high yields using a facile, extrusion-based methodology. Evaluation of biological properties reveals that LRNVs can promote neurogenesis and angiogenesis through modulation of lipid raft-dependent signaling pathways. A proof-of-concept methodology further shows that LRNVs could be loaded with proteins or other bioactive cargo for greater disease-specific functionalities, thus presenting a novel type of biomimetic nanovesicles that can be leveraged as targeted therapeutics for regenerative medicine.
Keywords: angiogenesis; drug delivery; extracellular vesicles; lipid rafts; neuroregeneration.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Slaughter N.; Laux I.; Tu X.; Whitelegge J.; Zhu X.; Effros R.; Bickel P.; Nel A. The Flotillins Are Integral Membrane Proteins in Lipid Rafts That Contain TCR-Associated Signaling Components: Implications for T-Cell Activation. Clin. Immunol. 2003, 108, 138–151. 10.1016/S1521-6616(03)00097-4. - DOI - PubMed

