Osteoarticular Mycoses
- PMID: 36448782
- PMCID: PMC9769674
- DOI: 10.1128/cmr.00086-19
Osteoarticular Mycoses
Abstract
Osteoarticular mycoses are chronic debilitating infections that require extended courses of antifungal therapy and may warrant expert surgical intervention. As there has been no comprehensive review of these diseases, the International Consortium for Osteoarticular Mycoses prepared a definitive treatise for this important class of infections. Among the etiologies of osteoarticular mycoses are Candida spp., Aspergillus spp., Mucorales, dematiaceous fungi, non-Aspergillus hyaline molds, and endemic mycoses, including those caused by Histoplasma capsulatum, Blastomyces dermatitidis, and Coccidioides species. This review analyzes the history, epidemiology, pathogenesis, clinical manifestations, diagnostic approaches, inflammatory biomarkers, diagnostic imaging modalities, treatments, and outcomes of osteomyelitis and septic arthritis caused by these organisms. Candida osteomyelitis and Candida arthritis are associated with greater events of hematogenous dissemination than those of most other osteoarticular mycoses. Traumatic inoculation is more commonly associated with osteoarticular mycoses caused by Aspergillus and non-Aspergillus molds. Synovial fluid cultures are highly sensitive in the detection of Candida and Aspergillus arthritis. Relapsed infection, particularly in Candida arthritis, may develop in relation to an inadequate duration of therapy. Overall mortality reflects survival from disseminated infection and underlying host factors.
Keywords: antifungal therapy; aspergillosis; candidiasis; coccidioidomycosis; cryptococcosis; histoplasmosis; mucormycosis; mycoses; osteomyelitis; phaeohyphomycosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
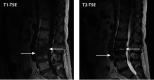
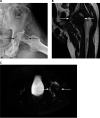


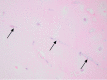

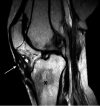

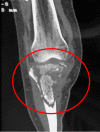
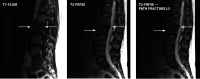
References
-
- Gamaletsou MN, Rammaert B, Bueno MA, Sipsas NV, Moriyama B, Kontoyiannis DP, Roilides E, Zeller V, Taj-Aldeen SJ, Miller AO, Petraitiene R, Lortholary O, Walsh TJ. 2016. Candida arthritis: analysis of 112 pediatric and adult cases. Open Forum Infect Dis 3:ofv207. 10.1093/ofid/ofv207. - DOI - PMC - PubMed
-
- Fuzibet JG, Squara P, Verdier JM, Lapalus P, Gratecos N, Cassuto JP, Chichmanian RM, Dujardin P. 1982. Candida albicans spondylitis—a case report with a study of bone penetration of 5-fluorocytosine and a review of the literature. Ann Med Interne (Paris) 133:410–415. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

