Randomized controlled trial of intrathecal oxytocin on speed of recovery after hip arthroplasty
- PMID: 36448974
- PMCID: PMC10106358
- DOI: 10.1097/j.pain.0000000000002810
Randomized controlled trial of intrathecal oxytocin on speed of recovery after hip arthroplasty
Abstract
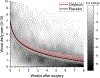
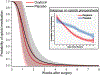
Recovery from surgery is quicker in the postpartum period, and this may reflect oxytocin action in the spinal cord. We hypothesized that intrathecal injection of oxytocin would speed recovery from pain and disability after major surgery. Ninety-eight individuals undergoing elective total hip arthroplasty were randomized to receive either intrathecal oxytocin (100 μg) or saline. Participants completed diaries assessing pain and opioid use daily and disability weekly, and they wore an accelerometer beginning 2 weeks before surgery until 8 weeks after. Groups were compared using modelled, adjusted trajectories of these measures. The study was stopped early due to the lack of funding. Ninety patients received intrathecal oxytocin (n = 44) or saline (n = 46) and were included in the analysis. There were no study drug-related adverse effects. Modelled pain trajectory, the primary analysis, did not differ between the groups, either in pain on day of hospital discharge (intercept: -0.1 [95% CI: -0.8 to 0.6], P = 0.746) or in reductions over time (slope: 0.1 pain units per log of time [95% CI: 0-0.2], P = 0.057). In planned secondary analyses, postoperative opioid use ended earlier in the oxytocin group and oxytocin-treated patients walked nearly 1000 more steps daily at 8 weeks ( P < 0.001) and exhibited a clinically meaningful reduction in disability for the first 21 postoperative days ( P = 0.007) compared with saline placebo. Intrathecal oxytocin before hip replacement surgery does not speed recovery from worst daily pain. Secondary analyses suggest that further study of intrathecal oxytocin to speed functional recovery without worsening pain after surgery is warranted.
Trial registration: ClinicalTrials.gov NCT00301130.
Copyright © 2022 International Association for the Study of Pain.
Conflict of interest statement
Declaration of Interests.
The authors declare no conflicts of interest.
Figures

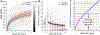
References
-
- Anderson KO, Dowds BN, Pelletz RE, Edwards WT, Peeters-Asdourian C. Development and initial validation of a scale to measure self-efficacy beliefs in patients with chronic pain. Pain 1995;63(1):77–84. - PubMed
-
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition 1994;50(1-3):7–15. - PubMed
-
- Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. The Journal of rheumatology 1988;15(12):1833–1840. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

