Development of a Clinical Prediction Model for 1-Year Mortality in Patients With Advanced Cancer
- PMID: 36449290
- PMCID: PMC9713606
- DOI: 10.1001/jamanetworkopen.2022.44350
Development of a Clinical Prediction Model for 1-Year Mortality in Patients With Advanced Cancer
Abstract
Importance: To optimize palliative care in patients with cancer who are in their last year of life, timely and accurate prognostication is needed. However, available instruments for prognostication, such as the surprise question ("Would I be surprised if this patient died in the next year?") and various prediction models using clinical variables, are not well validated or lack discriminative ability.
Objective: To develop and validate a prediction model to calculate the 1-year risk of death among patients with advanced cancer.
Design, setting, and participants: This multicenter prospective prognostic study was performed in the general oncology inpatient and outpatient clinics of 6 hospitals in the Netherlands. A total of 867 patients were enrolled between June 2 and November 22, 2017, and followed up for 1 year. The primary analyses were performed from October 9 to 25, 2019, with the most recent analyses performed from June 19 to 22, 2022. Cox proportional hazards regression analysis was used to develop a prediction model including 3 categories of candidate predictors: clinician responses to the surprise question, patient clinical characteristics, and patient laboratory values. Data on race and ethnicity were not collected because most patients were expected to be of White race and Dutch ethnicity, and race and ethnicity were not considered as prognostic factors. The models' discriminative ability was assessed using internal-external validation by study hospital and measured using the C statistic. Patients 18 years and older with locally advanced or metastatic cancer were eligible. Patients with hematologic cancer were excluded.
Main outcomes and measures: The risk of death by 1 year.
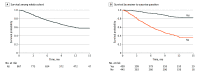
Results: Among 867 patients, the median age was 66 years (IQR, 56-72 years), and 411 individuals (47.4%) were male. The 1-year mortality rate was 41.6% (361 patients). Three prediction models with increasing complexity were developed: (1) a simple model including the surprise question, (2) a clinical model including the surprise question and clinical characteristics (age, cancer type prognosis, visceral metastases, brain metastases, Eastern Cooperative Oncology Group performance status, weight loss, pain, and dyspnea), and (3) an extended model including the surprise question, clinical characteristics, and laboratory values (hemoglobin, C-reactive protein, and serum albumin). The pooled C statistic was 0.69 (95% CI, 0.67-0.71) for the simple model, 0.76 (95% CI, 0.73-0.78) for the clinical model, and 0.78 (95% CI, 0.76-0.80) for the extended model. A nomogram and web-based calculator were developed to support clinicians in adequately caring for patients with advanced cancer.
Conclusions and relevance: In this study, a prediction model including the surprise question, clinical characteristics, and laboratory values had better discriminative ability in predicting death among patients with advanced cancer than models including the surprise question, clinical characteristics, or laboratory values alone. The nomogram and web-based calculator developed for this study can be used by clinicians to identify patients who may benefit from palliative care and advance care planning. Further exploration of the feasibility and external validity of the model is needed.
Conflict of interest statement
Figures

References
-
- Rietjens JAC, Sudore RL, Connolly M, et al. ; European Association for Palliative Care . Definition and recommendations for advance care planning: an international consensus supported by the European Association for Palliative Care. Lancet Oncol. 2017;18(9):e543-e551. doi:10.1016/S1470-2045(17)30582-X - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

