A Scoping Review and Meta-analysis of the Use of Remote Biochemical Verification Methods of Smoking Status in Tobacco Research
- PMID: 36449414
- PMCID: PMC10347976
- DOI: 10.1093/ntr/ntac271
A Scoping Review and Meta-analysis of the Use of Remote Biochemical Verification Methods of Smoking Status in Tobacco Research
Abstract
Introduction: Increasing digital delivery of smoking cessation interventions has resulted in the need to employ novel strategies for remote biochemical verification.
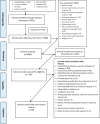
Aims and methods: This scoping review and meta-analysis aimed to investigate best practices for remote biochemical verification of smoking status. The scientific literature was searched for studies that reported remotely obtained (not in-person) biochemical confirmation of smoking status (ie, combustible tobacco). A meta-analysis of proportions was conducted to investigate key outcomes, which included rates of returned biological samples and the ratio of biochemically verified to self-reported abstinence rates.
Results: A total of 82 studies were included. The most common samples were expired air (46%) and saliva (40% of studies), the most common biomarkers were carbon monoxide (48%) and cotinine (44%), and the most common verification methods were video confirmation (37%) and mail-in samples for lab analysis (26%). Mean sample return rates determined by random-effects meta-analysis were 70% for smoking cessation intervention studies without contingency management (CM), 77% for CM studies, and 65% for other studies (eg, feasibility and secondary analyses). Among smoking cessation intervention studies without CM, self-reported abstinence rates were 21%, biochemically verified abstinence rates were 10%, and 47% of individuals who self-reported abstinence were also biochemically confirmed as abstinent.
Conclusions: This scoping review suggests that improvements in sample return rates in remote biochemical verification studies of smoking status are needed. Recommendations for reporting standards are provided that may enhance confidence in the validity of reported abstinence rates in remote studies.
Implications: This scoping review and meta-analysis included studies using remote biochemical verification to determine smoking status. Challenges exist regarding implementation and ensuring high sample return rates. Higher self-reported compared to biochemically verified abstinence rates suggest the possibility that participants in remote studies may be misreporting abstinence or not returning samples for other reasons (eg, participant burden, inconvenience). Remote biochemical confirmation of self-reported smoking abstinence should be included in smoking cessation studies whenever feasible. However, findings should be considered in the context of challenges to sample return rates. Better reporting guidelines for future studies in this area are needed.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Society for Research on Nicotine and Tobacco. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
Figures
References
-
- Prutzman YM, Wiseman KP, Grady MA, et al. Using digital technologies to reach tobacco users who want to quit: evidence from the national cancer institute’s smokefree.gov initiative. Am J Prev Med. 2021;60(3 suppl 2):S172–S184. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous