Effects of Conformational Constraint on Peptide Solubility Limits
- PMID: 36450134
- PMCID: PMC10270293
- DOI: 10.1021/acs.jpcb.2c06458
Effects of Conformational Constraint on Peptide Solubility Limits
Abstract
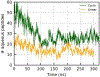
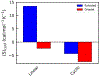
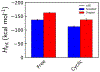
Liquid-liquid phase separation of proteins preferentially involves intrinsically disordered proteins or disordered regions. Understanding the solution chemistry of these phase separations is key to learning how to quantify and manipulate systems that involve such processes. Here, we investigate the effect of cyclization on the liquid-liquid phase separation of short polyglycine peptides. We simulated separate aqueous systems of supersaturated cyclic and linear GGGGG and observed spontaneous liquid-liquid phase separation in each of the solutions. The cyclic GGGGG phase separates less robustly than linear GGGGG and has a higher aqueous solubility, even though linear GGGGG has a more favorable single molecule solvation free energy. The versatile and abundant interpeptide contacts formed by the linear GGGGG stabilize the condensed droplet phase, driving the phase separation in this system. In particular, we find that van der Waals close contact interactions are enriched in the droplet phase as opposed to electrostatic interactions. An analysis of the change in backbone conformational entropy that accompanies the phase transition revealed that cyclic peptides lose significantly less entropy in this process as expected. However, we find that the enhanced interaction enthalpy of linear GGGGG in the droplet phase is enough to compensate for a larger decrease in conformational entropy.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
Thermodynamic Compensation in Peptides Following Liquid-Liquid Phase Separation.J Phys Chem B. 2021 Jun 24;125(24):6431-6439. doi: 10.1021/acs.jpcb.1c02093. Epub 2021 Jun 10. J Phys Chem B. 2021. PMID: 34110175 Free PMC article.
-
Thermodynamics of π-π Interactions of Benzene and Phenol in Water.Int J Mol Sci. 2022 Aug 29;23(17):9811. doi: 10.3390/ijms23179811. Int J Mol Sci. 2022. PMID: 36077201 Free PMC article.
-
Thermodynamic contribution of backbone conformational entropy in the binding between SH3 domain and proline-rich motif.Biochem Biophys Res Commun. 2017 Feb 26;484(1):21-26. doi: 10.1016/j.bbrc.2017.01.089. Epub 2017 Jan 19. Biochem Biophys Res Commun. 2017. PMID: 28111343
-
Distinct role of hydration water in protein misfolding and aggregation revealed by fluctuating thermodynamics analysis.Acc Chem Res. 2015 Apr 21;48(4):956-65. doi: 10.1021/acs.accounts.5b00032. Epub 2015 Apr 6. Acc Chem Res. 2015. PMID: 25844814 Review.
-
Intrinsically disordered protein regions and phase separation: sequence determinants of assembly or lack thereof.Emerg Top Life Sci. 2020 Dec 11;4(3):307-329. doi: 10.1042/ETLS20190164. Emerg Top Life Sci. 2020. PMID: 33078839 Review.
Cited by
-
Developing an Implicit Solvation Machine Learning Model for Molecular Simulations of Ionic Media.J Chem Theory Comput. 2024 Jan 9;20(1):411-420. doi: 10.1021/acs.jctc.3c00984. Epub 2023 Dec 20. J Chem Theory Comput. 2024. PMID: 38118122 Free PMC article.
-
Peptide diffusion in biomolecular condensates.Biophys J. 2024 Jun 18;123(12):1668-1675. doi: 10.1016/j.bpj.2024.05.009. Epub 2024 May 15. Biophys J. 2024. PMID: 38751116 Free PMC article.
References
-
- Uversky VN Intrinsically disordered proteins in overcrowded milieu: Membrane-less organelles, phase separation, and intrinsic disorder. Curr. Opin. Struct. Biol 2017, 44, 18–30. - PubMed
-
- Falahati H; Haji-Akbari A Thermodynamically driven assemblies and liquid–liquid phase separations in biology. Soft Matter 2019, 15, 1135–1154. - PubMed
-
- Hondele M; Heinrich S; De Los Rios P; Weis K Membraneless organelles: phasing out of equilibrium. Emerging Topics in Life Sciences 2020, 4, 343–354. - PubMed
-
- Hyman AA; Weber CA; Jülicher F Liquid-Liquid Phase Separation in Biology. Annual Review of Cell and Developmental Biology 2014, 30, 39–58. - PubMed
-
- Alberti S; Dormann D Liquid–Liquid Phase Separation in Disease. Annual Review of Genetics 2019, 53, 171–194. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

