Immune checkpoint inhibitor therapy and outcomes from SARS-CoV-2 infection in patients with cancer: a joint analysis of OnCovid and ESMO-CoCARE registries
- PMID: 36450384
- PMCID: PMC9716413
- DOI: 10.1136/jitc-2022-005732
Immune checkpoint inhibitor therapy and outcomes from SARS-CoV-2 infection in patients with cancer: a joint analysis of OnCovid and ESMO-CoCARE registries
Abstract
Background: As management and prevention strategies against COVID-19 evolve, it is still uncertain whether prior exposure to immune checkpoint inhibitors (ICIs) affects COVID-19 severity in patients with cancer.
Methods: In a joint analysis of ICI recipients from OnCovid (NCT04393974) and European Society for Medical Oncology (ESMO) CoCARE registries, we assessed severity and mortality from SARS-CoV-2 in vaccinated and unvaccinated patients with cancer and explored whether prior immune-related adverse events (irAEs) influenced outcome from COVID-19.
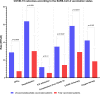
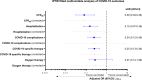
Findings: The study population consisted of 240 patients diagnosed with COVID-19 between January 2020 and February 2022 exposed to ICI within 3 months prior to COVID-19 diagnosis, with a 30-day case fatality rate (CFR30) of 23.6% (95% CI 17.8 to 30.7%). Overall, 42 (17.5%) were fully vaccinated prior to COVID-19 and experienced decreased CFR30 (4.8% vs 28.1%, p=0.0009), hospitalization rate (27.5% vs 63.2%, p<0.0001), requirement of oxygen therapy (15.8% vs 41.5%, p=0.0030), COVID-19 complication rate (11.9% vs 34.6%, p=0.0040), with a reduced need for COVID-19-specific therapy (26.3% vs 57.9%, p=0.0004) compared with unvaccinated patients. Inverse probability of treatment weighting (IPTW)-fitted multivariable analysis, following a clustered-robust correction for the data source (OnCovid vs ESMO CoCARE), confirmed that vaccinated patients experienced a decreased risk of death at 30 days (adjusted OR, aOR 0.08, 95% CI 0.01 to 0.69).Overall, 38 patients (15.8%) experienced at least one irAE of any grade at any time prior to COVID-19, at a median time of 3.2 months (range 0.13-48.7) from COVID-19 diagnosis. IrAEs occurred independently of baseline characteristics except for primary tumor (p=0.0373) and were associated with a significantly decreased CFR30 (10.8% vs 26.0%, p=0.0462) additionally confirmed by the IPTW-fitted multivariable analysis (aOR 0.47, 95% CI 0.33 to 0.67). Patients who experienced irAEs also presented a higher median absolute lymphocyte count at COVID-19 (1.4 vs 0.8 109 cells/L, p=0.0098).
Conclusion: Anti-SARS-CoV-2 vaccination reduces morbidity and mortality from COVID-19 in ICI recipients. History of irAEs might identify patients with pre-existing protection from COVID-19, warranting further investigation of adaptive immune determinants of protection from SARS-CoV-2.
Keywords: COVID-19; Cytotoxicity, Immunologic; Immunogenicity, Vaccine; Immunotherapy; Vaccination.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: KJH declares research funding from AstraZeneca, Boehringer-Ingelheim, MSD, Replimune and advisory board fees/honoraria from Arch Oncology, AstraZeneca, BMS, Boehringer-Ingelheim, Codiak Biosciences, Inzen Therapeutics, Merck-Serono, MSD, Pfizer, Replimune. DA reports consultation/advisory role for AstraZeneca, Bristol Myers Squibb, Merck Sharp reports speaker’s engagement from AstraZeneca, Bristol Myers Squibb, Merck Sharp reports serving as local PI for Bristol Myers Squibb, Pierre Fabre Pharma and coordinating PI for OncoLytics; reports grant funding from AbbVie; reports being/been DSMB chair of Sanofi (Genzyme); reports being/been a steering committee member of Roche. Olivier Michielin reports personal fees from Bristol-Myers Squibb, MSD, Novartis, Roche, Amgen, NeraCare, outside the submitted work. JR received speaker or advisory fees from Roche, Astra Zeneca, Merck, Ferrer, Persan Farma, Teva Pharma, Leo Pharma, Fresenius kabi, MSD, BMS. Travel expenses support from BMS, MSD, RocheUrania Dafni reports honorarium as Member of the Tumor Agnostic Evidence Generation Working Group of Roche, outside the submitted work. GP reports grants from Amgen, Lilly; grants, personal fees and nonfinancial support from Merck; grants and non-financial support from AstraZeneca; grants and personal fees from Roche, Bristol Myers Squibb, MSD, Novartis, outside the submitted work. Solange Peters reports consultation/advisory role for AbbVie, Amgen, AstraZeneca, Bayer, BeiGene, Biocartis, Bio Invent, Blueprint Medicines, Boehringer Ingelheim, Bristol- Myers Squibb, Clovis, Daiichi Sankyo, Debiopharm, Eli Lilly, Elsevier, F Hoffmann-La Roche/Genentech, Foundation Medicine, Illumina, Incyte, IQVIA, Janssen, Medscape, Merck Sharp and Dohme, Merck Serono, Merrimack, Mirati, Novartis, Pharma Mar, Phosplatin Therapeutics, Pfizer, Regeneron, Sanofi, Seattle Genetics, Takeda, Vaccibody; talk in a company’s organized public event for AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, e-cancer, Eli Lilly, F. Hoffmann-La Roche/Genentech, Illumina, Medscape, Merck Sharp and Dohme, Novartis, PER, Pfizer, Prime, RTP, Sanofi, Takeda; receipt of grants/research supports from being a (sub)investigator in trials (institutional financial support for clinical trials) sponsored by Amgen, AstraZeneca, Biodesix, Boehringer Ingelheim, Bristol-Myers Squibb, Clovis, F Hoffmann-La Roche/Genentech, GSK, Illumina, Lilly, Merck Sharp and Dohme, Merck Serono, Mirati, Novartis, and Pfizer, Phosplatin Therapeutics. Emanuela Rromano reports investigator-initiated trial (funds paid to the institution) supported by Astra-Zeneca, BMS; serves on the consultancy/advisory board for Astra-Zeneca, Merck, Roche, Pierre Fabre. ML acted as consultant for Roche, Novartis, Lilly, AstraZeneca, Exact Sciences, MSD, Pfizer, Seagen and received speaker honoraria from Roche, Novartis, Lilly, Pfizer, Takeda, Ipsen and Sandoz outside the submitted work.Alessandra Gennari has declared consulting/advisory role for Roche, MSD, Eli Lilly, Pierre Fabre, EISAI, and Daichii Sankyo; speakers bureau for Eisai, Novartis, Eli Lilly, Roche, Teva, Gentili, Pfizer, Astra Zeneca, Celgene, and Daichii Sankyo; research funds: EISAI, Eli Lilly, and Roche. CMV has received travel grants and other honoraria from BMS, MSD, Novartis and Roche. Joan Brunet has declared consulting/advisory role for MSD and Astra Zeneca. Aleix Prat has declared personal honoraria from Pfizer, Roche, MSD Oncology, Eli Lilly, and Daiichi Sankyo; travel, accommodations, and expenses paid by Daiichi Sankyo; research funding from Roche and Novartis; and consulting/advisory role for NanoString Technologies, Amgen, Roche, Novartis, Pfizer and Bristol-Myers Squibb. Mark Bower received speakers’ fee from EISAI pharma, Gilead Sciences, Merck and ViiV. JT reports consulting fees from Array Biopharma, AstraZeneca, Avvinity, Bayer, Boehringer Ingelheim, Chugai, Daiichi Sankyo, F Hoffmann-La Roche, Genentech, HalioDX SAS, Hutchison MediPharma International, Ikena Oncology, Inspirna, IQVIA, Lilly, Menarini, Merck Serono, Merus, MSD, Mirati, Neophore, Novartis, Ona Therapeutics, Orion Biotechnology, Peptomyc, Pfizer, Pierre Fabre, Samsung Bioepis, Sanofi, Seattle Genetics, Scandion Oncology, Servier, Sotio Biotech, Taiho, Tessa Therapeutics, and TheraMyc; speaker’s fees from Imedex, Medscape Education, MJH Life Sciences, PeerView Institute for Medical Education, and Physicians Education Resource; and institutional research support from Amgen, Array Biopharma, AstraZeneca Pharmaceuticals, BeiGene, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Debiopharm International, F Hoffmann-La Roche, Genentech, HalioDX, Hutchison MediPharma International, Janssen-Cilag, MedImmune, Menarini, Merck Health, Merck Sharp and has had leadership roles in the European AIDS Clinical Society, UNAIDS, WHO, and The European Hematology Association/ European Society of Medical Oncology. DJP received lecture fees from ViiV Healthcare, Bayer Healthcare, BMS, Roche, EISAI, Falk Foundation, travel expenses from BMS and Bayer Healthcare; consulting fees for Mina Therapeutics, EISAI, Roche, DaVolterra and Astra Zeneca; research funding (to institution) from MSD and BMS. AC received consulting fees from MSD, BMS, AstraZeneca, Roche; speakers’ fee from AstraZeneca, MSD, Novartis and Eisai. All remaining authors have declared no conflicts of interest.
Figures


Similar articles
-
COVID-19 in cancer patients: update from the joint analysis of the ESMO-CoCARE, BSMO, and PSMO international databases.ESMO Open. 2023 Jun;8(3):101566. doi: 10.1016/j.esmoop.2023.101566. Epub 2023 May 4. ESMO Open. 2023. PMID: 37285719 Free PMC article.
-
Outcomes of the SARS-CoV-2 omicron (B.1.1.529) variant outbreak among vaccinated and unvaccinated patients with cancer in Europe: results from the retrospective, multicentre, OnCovid registry study.Lancet Oncol. 2022 Jul;23(7):865-875. doi: 10.1016/S1470-2045(22)00273-X. Epub 2022 Jun 2. Lancet Oncol. 2022. PMID: 35660139 Free PMC article.
-
Vaccination against SARS-CoV-2 protects from morbidity, mortality and sequelae from COVID19 in patients with cancer.Eur J Cancer. 2022 Aug;171:64-74. doi: 10.1016/j.ejca.2022.04.036. Epub 2022 May 23. Eur J Cancer. 2022. PMID: 35704976 Free PMC article.
-
COVID-19 vaccination in patients with cancer receiving immune checkpoint inhibitors: a systematic review and meta-analysis.J Immunother Cancer. 2023 Feb;11(2):e006246. doi: 10.1136/jitc-2022-006246. J Immunother Cancer. 2023. PMID: 36746512 Free PMC article.
-
Influenza vaccination in cancer patients receiving immune checkpoint inhibitors: A systematic review.Eur J Clin Invest. 2021 Jul;51(7):e13604. doi: 10.1111/eci.13604. Epub 2021 May 31. Eur J Clin Invest. 2021. PMID: 34021591 Free PMC article.
Cited by
-
COVID-19 vaccination is associated with enhanced efficacy of anti-PD-(L)1 immunotherapy in advanced NSCLC patients: a real-world study.Infect Agent Cancer. 2023 Sep 7;18(1):50. doi: 10.1186/s13027-023-00526-7. Infect Agent Cancer. 2023. PMID: 37679851 Free PMC article.
-
Association of immune-related adverse events with COVID-19 pneumonia in lung cancer patients receiving immune checkpoint inhibitors: a cross-sectional study in China.BMC Cancer. 2023 Nov 6;23(1):1069. doi: 10.1186/s12885-023-11584-w. BMC Cancer. 2023. PMID: 37932685 Free PMC article.
-
Immune checkpoint inhibitors and risk of immune-mediated adverse events: a cohort study comparing extended versus standard interval administration.Clin Exp Med. 2024 Feb 22;24(1):40. doi: 10.1007/s10238-024-01301-7. Clin Exp Med. 2024. PMID: 38386053 Free PMC article.
-
Is COVID-19 Still a Threat? An Expert Opinion Review on the Continued Healthcare Burden in Immunocompromised Individuals.Adv Ther. 2025 Feb;42(2):666-719. doi: 10.1007/s12325-024-03043-0. Epub 2024 Dec 16. Adv Ther. 2025. PMID: 39680311 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
