Active and dormant microorganisms on glacier surfaces
- PMID: 36450703
- PMCID: PMC10099831
- DOI: 10.1111/gbi.12535
Active and dormant microorganisms on glacier surfaces
Abstract
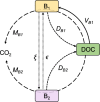
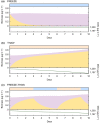
Glacier and ice sheet surfaces host diverse communities of microorganisms whose activity (or inactivity) influences biogeochemical cycles and ice melting. Supraglacial microbes endure various environmental extremes including resource scarcity, frequent temperature fluctuations above and below the freezing point of water, and high UV irradiance during summer followed by months of total darkness during winter. One strategy that enables microbial life to persist through environmental extremes is dormancy, which despite being prevalent among microbial communities in natural settings, has not been directly measured and quantified in glacier surface ecosystems. Here, we use a combination of metabarcoding and metatranscriptomic analyses, as well as cell-specific activity (BONCAT) incubations to assess the diversity and activity of microbial communities from glacial surfaces in Iceland and Greenland. We also present a new ecological model for glacier microorganisms and simulate physiological state-changes in the glacial microbial community under idealized (i) freezing, (ii) thawing, and (iii) freeze-thaw conditions. We show that a high proportion (>50%) of bacterial cells are translationally active in-situ on snow and ice surfaces, with Actinomycetota, Pseudomonadota, and Planctomycetota dominating the total and active community compositions, and that glacier microorganisms, even when frozen, could resume translational activity within 24 h after thawing. Our data suggest that glacial microorganisms respond rapidly to dynamic and changing conditions typical of their natural environment. We deduce that the biology and biogeochemistry of glacier surfaces are shaped by processes occurring over short (i.e., daily) timescales, and thus are susceptible to change following the expected alterations to the melt-regime of glaciers driven by climate change. A better understanding of the activity of microorganisms on glacier surfaces is critical in addressing the growing concern of climate change in Polar regions, as well as for their use as analogues to life in potentially habitable icy worlds.
Keywords: activity; dormancy; extremophiles; glacier; ice; microorganisms; snow.
© 2022 The Authors. Geobiology published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- AMAP . (2021). Arctic climate change update 2021: Key trends and impacts . Summary for Policy‐makers. Arctic Monitoring and Assessment Programme (AMAP).
-
- Anesio, A. M. , Hodson, A. J. , Fritz, A. , Psenner, R. , & Sattler, B. (2009). High microbial activity on glaciers: Importance to the global carbon cycle. Global Change Biology, 15, 955–960. 10.1111/j.1365-2486.2008.01758.x - DOI
-
- Anesio, A. M. , Sattler, B. , Foreman, C. , Telling, J. , Hodson, A. , Tranter, M. , & Psenner, R. (2010). Carbon fluxes through bacterial communities on glacier surfaces. Annals of Glaciology, 51, 32–40.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

