Multiple invasions, Wolbachia and human-aided transport drive the genetic variability of Aedes albopictus in the Iberian Peninsula
- PMID: 36450768
- PMCID: PMC9712423
- DOI: 10.1038/s41598-022-24963-3
Multiple invasions, Wolbachia and human-aided transport drive the genetic variability of Aedes albopictus in the Iberian Peninsula
Abstract
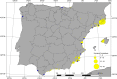
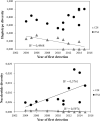
The Asian tiger mosquito, Aedes albopictus, is one of the most invasive species in the world. Native to the tropical forests of Southeast Asia, over the past 30 years it has rapidly spread throughout tropical and temperate regions of the world. Its dramatic expansion has resulted in public health concerns as a consequence of its vector competence for at least 16 viruses. Previous studies showed that Ae. albopictus spread has been facilitated by human-mediated transportation, but much remains unknown about how this has affected its genetic attributes. Here we examined the factors that contributed to shaping the current genetic constitution of Ae. albopictus in the Iberian Peninsula, where the species was first found in 2004, by combining population genetics and Bayesian modelling. We found that both mitochondrial and nuclear DNA markers showed a lack of genetic structure and the presence of worldwide dominant haplotypes, suggesting regular introductions from abroad. Mitochondrial DNA showed little genetic diversity compared to nuclear DNA, likely explained by infection with maternally transmitted bacteria of the genus Wolbachia. Multilevel models revealed that greater mosquito fluxes (estimated from commuting patterns and tiger mosquito population distribution) and spatial proximity between sampling sites were associated with lower nuclear genetic distance, suggesting that rapid short- and medium-distance dispersal is facilitated by humans through vehicular traffic. This study highlights the significant role of human transportation in shaping the genetic attributes of Ae. albopictus and promoting regional gene flow, and underscores the need for a territorially integrated surveillance across scales of this disease-carrying mosquito.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Hawley WA. The biology of Aedes albopictus. J. Am. Mosq. Control Assoc. 1988;1:1–39. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

