Valuation of Treatments for Rare Diseases: A Systematic Literature Review of Societal Preference Studies
- PMID: 36451072
- PMCID: PMC9898379
- DOI: 10.1007/s12325-022-02359-z
Valuation of Treatments for Rare Diseases: A Systematic Literature Review of Societal Preference Studies
Abstract
Introduction: We sought to synthesize published empirical studies that elicited and characterized societal valuations of orphan drugs and the attributes that may drive different valuations for orphan drugs versus other treatments.
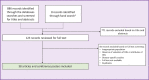
Methods: We conducted a systematic literature review (SLR) in MEDLINE and EMBASE databases up to November 2, 2020. Search terms covered societal preferences and attributes of orphan drugs (e.g., disease prevalence, severity, burden, unmet needs, and benefits).
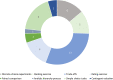
Results: We identified 38 eligible publications: 33 societal preference studies and 5 reviews discussing societal valuations and attributes of orphan drugs. Most publications suggested that a majority of respondents favored allocating funds to more prevalent diseases. However, trade-off studies and discrete-choice experiments found that survey participants chose to allocate resources to orphan drugs even when the cost per unit of health benefit was greater than for therapies for more prevalent diseases. Overall, 19 of 27 studies assessing severity in treatment valuation revealed that respondents prioritized patients with severe diseases over those with milder ones for equal health benefits. Members of the general public tended to prefer treatments for diseases with no alternative or when existing alternatives had limited efficacy over diseases with clear therapeutic alternatives. There was evidence that individuals preferred sharing resources, so no patient was left without treatment.
Conclusions: Our SLR indicates the general public typically attaches greater value to orphan drugs than to other treatments for common diseases. This is not because of rarity per se, but primarily because of disease severity and lack of therapeutic alternatives typically associated with rare diseases.
Keywords: Orphan drugs; Rare diseases; Societal preferences; Systematic literature review; Treatments for rare diseases; Valuation.
Plain language summary
Orphan drugs are drugs serving a substantial public health need by treating life-threatening or chronically debilitating medical conditions affecting a small number of people with very high unmet needs. We reviewed 38 published studies looking at drug characteristics that may cause people to value orphan drugs differently versus treatments for common conditions. Most people surveyed in these publications favored health care funds going to more prevalent diseases. However, some people preferred funding orphan drugs even when the cost versus health benefit was higher compared with treatments for more common diseases. The majority of studies that investigated the impact of disease severity on the valuation of treatments found that people prioritized patients with severe disease over those with milder disease, for the same extent of health benefit. People also preferred funding treatments for diseases that have no alternative treatments, or treatments with limited benefits, over treatments for diseases with many treatments or more effective treatments. We also found evidence of a societal preference for shared resources, meaning that no patient would be left without treatment, including those who receive limited benefits from health care resources, even if this does not lead to the maximization of health benefits across society. In conclusion, our literature review indicated that the general public attaches greater value to orphan drugs versus treatments for more common diseases, not because of rarity per se, but largely because the rare diseases treated by orphan drugs are often severe and have no or few treatment options.
© 2022. The Author(s).
Figures

References
-
- European Medicines Agency. Orphan designation: overview. https://www.ema.europa.eu/en/human-regulatory/overview/orphan-designatio.... Accessed 10 June 2021.
-
- Kesselheim AS. B, Innovation and the Orphan Drug Act, 1983–2009: regulatory and clinical characteristics of approved orphan drugs. In: Field MJ, Boat TF, editors. Rare diseases and orphan products: accelerating research and development. Washington, DC: National Academies Press (US); 2010. pp. 291–308. - PubMed
-
- Swann J. The story behind the Orphan Drug Act. 2018. https://www.fda.gov/industry/orphan-products-development-events/story-be.... Accessed 15 Feb 2022.
-
- European Medicines Agency. Legal framework: orphan designation. https://www.ema.europa.eu/en/human-regulatory/overview/orphan-designatio.... Accessed 15 Feb 2022.
-
- Brown DG, Wobst HJ. A decade of FDA-approved drugs (2010–2019): trends and future directions. J Med Chem. 2021;64(5):2312–2338. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

