Use of proton pump inhibitors and macrolide antibiotics and risk of acute kidney injury: a self-controlled case series study
- PMID: 36451129
- PMCID: PMC9710142
- DOI: 10.1186/s12882-022-03008-x
Use of proton pump inhibitors and macrolide antibiotics and risk of acute kidney injury: a self-controlled case series study
Abstract
Background: Proton pump inhibitors (PPIs) are widely used for the treatment of gastrointestinal disorders such as peptic ulcer disease and dyspepsia. However, several studies have suggested that PPI use increases the risk of acute kidney injury (AKI). PPIs are often concomitantly used with antibiotics, such as macrolides and penicillins for Helicobacter pylori eradication. Although macrolide antibiotics are considered to have relatively low nephrotoxicity, they are well known to increase the risk of AKI due to drug-drug interactions. In this study, we aimed to investigate the association between PPI use and the development of AKI. We also evaluated the effect of concomitant use of PPIs and macrolide antibiotics on the risk of AKI.
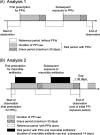
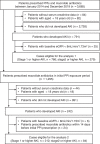
Methods: This self-controlled case series study was conducted using electronic medical records at Kyoto University Hospital. We identified patients who were prescribed at least one PPI and macrolide antibiotic between January 2014 and December 2019 and underwent blood examinations at least once a year. An adjusted incident rate ratio (aIRR) of AKI with PPI use or concomitant use macrolide antibiotics with PPIs was estimated using a conditional Poisson regression model controlled for the estimated glomerular filtration rate at the beginning of observation and use of potentially nephrotoxic antibiotics.
Results: Of the 3,685 individuals who received PPIs and macrolide antibiotics, 766 patients with episodes of stage 1 or higher AKI were identified. Any stage of AKI was associated with PPI use (aIRR, 1.80 (95% confidence interval (CI) 1.60 to 2.04)). Stage 2 or higher AKI was observed in 279 cases, with an estimated aIRR of 2.01 (95% CI 1.57 to 2.58, for PPI use). For the period of concomitant use of macrolide antibiotics with PPIs compared with the period of PPIs alone, an aIRR of stage 1 or higher AKI was estimated as 0.82 (95% CI 0.60 to 1.13).
Conclusions: Our findings added epidemiological information for the association between PPI use and an increased risk of stage 1 or higher AKI. However, we did not detect an association between the concomitant use of macrolide antibiotics and an increased risk of AKI in PPI users.
Keywords: Acute kidney injury; Macrolide antibiotics; Proton pump inhibitor; Self-controlled case series study.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests
Figures
Similar articles
-
Association of proton pump inhibitors and concomitant drugs with risk of acute kidney injury: a nested case-control study.BMJ Open. 2021 Feb 15;11(2):e041543. doi: 10.1136/bmjopen-2020-041543. BMJ Open. 2021. PMID: 33589451 Free PMC article.
-
Association between preoperative proton pump inhibitor use and postoperative acute kidney injury in patients undergoing major surgery.Ren Fail. 2024 Dec;46(2):2379596. doi: 10.1080/0886022X.2024.2379596. Epub 2024 Aug 4. Ren Fail. 2024. PMID: 39099235 Free PMC article.
-
Proton Pump Inhibitors and Risk of Acute and Chronic Kidney Disease: A Retrospective Cohort Study.Pharmacotherapy. 2019 Apr;39(4):443-453. doi: 10.1002/phar.2235. Epub 2019 Mar 21. Pharmacotherapy. 2019. PMID: 30779194 Free PMC article.
-
Systematic reviews of the clinical effectiveness and cost-effectiveness of proton pump inhibitors in acute upper gastrointestinal bleeding.Health Technol Assess. 2007 Dec;11(51):iii-iv, 1-164. doi: 10.3310/hta11510. Health Technol Assess. 2007. PMID: 18021578 Review.
-
Association of Proton Pump Inhibitor Use and Immune Checkpoint Inhibitor-Mediated Acute Kidney Injury: A Meta-Analysis and a Review of Related Outcomes.Am J Nephrol. 2024;55(4):439-449. doi: 10.1159/000538274. Epub 2024 Mar 12. Am J Nephrol. 2024. PMID: 38471492 Free PMC article.
Cited by
-
Impact of Proton Pump Inhibitors on Kidney Function and Chronic Kidney Disease Progression: A Systematic Review.Cureus. 2023 Dec 3;15(12):e49883. doi: 10.7759/cureus.49883. eCollection 2023 Dec. Cureus. 2023. PMID: 38174181 Free PMC article. Review.
-
Helicobacter pylori Eradication Therapy in Patients with Decreased Renal Function: A Systematic Review.J Clin Med. 2024 Feb 1;13(3):850. doi: 10.3390/jcm13030850. J Clin Med. 2024. PMID: 38337544 Free PMC article. Review.
-
Developing a novel magnetic organic polymer for selective extraction and determination of 16 macrolides in water and honey samples.RSC Adv. 2024 Mar 18;14(13):8726-8734. doi: 10.1039/d4ra00496e. eCollection 2024 Mar 14. RSC Adv. 2024. PMID: 38500629 Free PMC article.
-
Association between Proton Pump Inhibitors, Immune Checkpoint Inhibitors, and Acute Kidney Injury: A Nested Case-Control Study.Kidney360. 2024 Sep 1;5(9):1262-1269. doi: 10.34067/KID.0000000000000528. Epub 2024 Aug 1. Kidney360. 2024. PMID: 39088266 Free PMC article.
-
A Meta-Analysis of Proton Pump Inhibitor Use and the Risk of Acute Kidney Injury: Geographical Differences and Associated Factors.J Clin Med. 2023 Mar 24;12(7):2467. doi: 10.3390/jcm12072467. J Clin Med. 2023. PMID: 37048551 Free PMC article.

