Minimal clinically important difference for the Mandarin version of the Tinnitus Questionnaire determined via anchor-based and distribution-based methods
- PMID: 36451205
- PMCID: PMC9710156
- DOI: 10.1186/s12955-022-02072-z
Minimal clinically important difference for the Mandarin version of the Tinnitus Questionnaire determined via anchor-based and distribution-based methods
Abstract
Background: The previous study showed that the Mandarin Tinnitus Questionnaire (MTQ) has satisfactory reliability and validity. We have also completed the classification of the severity of tinnitus based on MTQ scores. In clinical studies, efficacy is often judged by whether results are statistically significant; however, statistical significance does not necessarily equate to clinical significance, whereas the minimum clinically important difference (MCID) of the scale does. In the following project, we will explore the MCID of the MTQ.
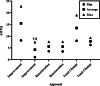
Methods: We recruited participants aged 18 years and above who sought treatment for primary or secondary tinnitus at the Otorhinolaryngology Department of the Hearing Center of West China Hospital, Sichuan University from September 2020 to September 2021. The participants had to undergo the following four assessments of tinnitus severity: doctor evaluation, self-report, the MTQ, and the visual analog scale (VAS), all at baseline and at the follow-up. The MCIDs of the MTQ were established via anchor-based and distribution-based methods. The anchor method used the VAS and self-reported clinical impression as anchors and defined the treatment effectiveness by mean/median and receiver operating characteristic (ROC) curve, while methods of effect size (ES), standard error of measurement (SEM), and reliability change index (RCI) were used in distribution-based methods.
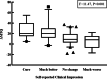
Results: A total of 115 patients were investigated in this study, 57.4% of whom were women. The average age was 43.2 ± 13.20 years. The average MTQ and VAS scores at baseline were 31.3 ± 14.90 and 5.03 ± 2.24, respectively, while the average MTQ and VAS scores at follow-up were 15.9 ± 11.70 and 3.58 ± 2.48, respectively. Moreover, in terms of self-reported clinical impressions, 19 patients indicated that they were cured (16.5%), 24 that it was much better (20.9%), 63 that there was no change (54.8%), and 9 that it was much worse (7.8%). The MCIDs for the change in total MTQ ranged from 6.29 to 19.00, those for improvement from 1.09 to 22.75, and those for deterioration from 3.50 to 7.64.
Conclusion: We selected an absolute value of 7.5 as the MCID for the MTQ score. An increase in MTQ score more than 7.5 was considered aggravation of tinnitus, and a decrease in MTQ score more than 7.5 was considered a reduction in tinnitus.
Keywords: Mandarin Tinnitus Questionnaire (MTQ); Minimal clinically important difference (MCID); Tinnitus.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Meng Z, Tao Y, Xu K, Li G, Zheng Y. Introduction of Mandarin version of the Tinnitus Questionnaire. J Audiol Speech Pathol. 2019;27:72–76.
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

