Biofilm Signaling, Composition and Regulation in Burkholderia pseudomallei
- PMID: 36451302
- PMCID: PMC9899790
- DOI: 10.4014/jmb.2207.07032
Biofilm Signaling, Composition and Regulation in Burkholderia pseudomallei
Abstract
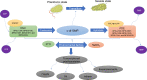
The incidence of melioidosis cases caused by the gram-negative pathogen Burkholderia pseudomallei (BP) is seeing an increasing trend that has spread beyond its previously known endemic regions. Biofilms produced by BP have been associated with antimicrobial therapy limitation and relapse melioidosis, thus making it urgently necessary to understand the mechanisms of biofilm formation and their role in BP biology. Microbial cells aggregate and enclose within a self-produced matrix of extracellular polymeric substances (EPSs) to form biofilm. The transition mechanism of bacterial cells from planktonic state to initiate biofilm formation, which involves the formation of surface attachment microcolonies and the maturation of the biofilm matrix, is a dynamic and complex process. Despite the emerging findings on the biofilm formation process, systemic knowledge on the molecular mechanisms of biofilm formation in BP remains fractured. This review provides insights into the signaling systems, matrix composition, and the biosynthesis regulation of EPSs (exopolysaccharide, eDNA and proteins) that facilitate the formation of biofilms in order to present an overview of our current knowledge and the questions that remain regarding BP biofilms.
Keywords: Burkholderia pseudomallei; biofilm; cyclic-di-GMP; eDNA; exopolysaccharide; quorum sensing.
Conflict of interest statement
The authors have no financial conflicts of interest to declare.
Figures

References
-
- Panomket P, Wongsana P, Wanram S, Wongratanacheewin S. Burkholderia pseudomallei biofilm plays a key role in chronic inflammation in C57BL/6 mice. Southeast Asian J. Trop. Med. Public Health. 2017;48:73–82. - PubMed

