Hospitalisation for COVID-19 predicts long lasting cerebrovascular impairment: A prospective observational cohort study
- PMID: 36451358
- PMCID: PMC9639388
- DOI: 10.1016/j.nicl.2022.103253
Hospitalisation for COVID-19 predicts long lasting cerebrovascular impairment: A prospective observational cohort study
Abstract
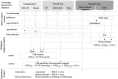
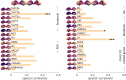
Human coronavirus disease 2019 (COVID-19) due to severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has multiple neurological consequences, but its long-term effect on brain health is still uncertain. The cerebrovascular consequences of COVID-19 may also affect brain health. We studied the chronic effect of COVID-19 on cerebrovascular health, in relation to acute severity, adverse clinical outcomes and in contrast to control group data. Here we assess cerebrovascular health in 45 patients six months after hospitalisation for acute COVID-19 using the resting state fluctuation amplitudes (RSFA) from functional magnetic resonance imaging, in relation to disease severity and in contrast with 42 controls. Acute COVID-19 severity was indexed by COVID-19 WHO Progression Scale, inflammatory and coagulatory biomarkers. Chronic widespread changes in frontoparietal RSFA were related to the severity of the acute COVID-19 episode. This relationship was not explained by chronic cardiorespiratory dysfunction, age, or sex. The level of cerebrovascular dysfunction was associated with cognitive, mental, and physical health at follow-up. The principal findings were consistent across univariate and multivariate approaches. The results indicate chronic cerebrovascular impairment following severe acute COVID-19, with the potential for long-term consequences on cognitive function and mental wellbeing.
Keywords: COVID-19; Cardiorespiratory; Cerebrovascular; Microvascular; Neurology; SARS-CoV-2.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





References
-
- Agarwal S., Sair H., Gujar S., Hua J., Lu H., Pillai J. Functional Magnetic Resonance Imaging Activation Optimization in the Setting of Brain Tumor-Induced Neurovascular Uncoupling Using Resting-State Blood Oxygen Level-Dependent Amplitude of Low Frequency Fluctuations. Brain Connect. 2019;9:241–250. doi: 10.1089/BRAIN.2017.0562. - DOI - PMC - PubMed
-
- Arnatkevic̆iūtė A., Fulcher B.D., Fornito A. A practical guide to linking brain-wide gene expression and neuroimaging data. Neuroimage. 2019;189:353–367. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

